2 KUTV
WASHINGTON POST
HEALTH 202: The newest way to test for coronavirus: Spitting in a tube
HEALTH 202: The newest way to test for coronavirus: Spitting in a tube
by Jennifer Weaver
Friday, April 3rd 2020
Friday, April 3rd 2020
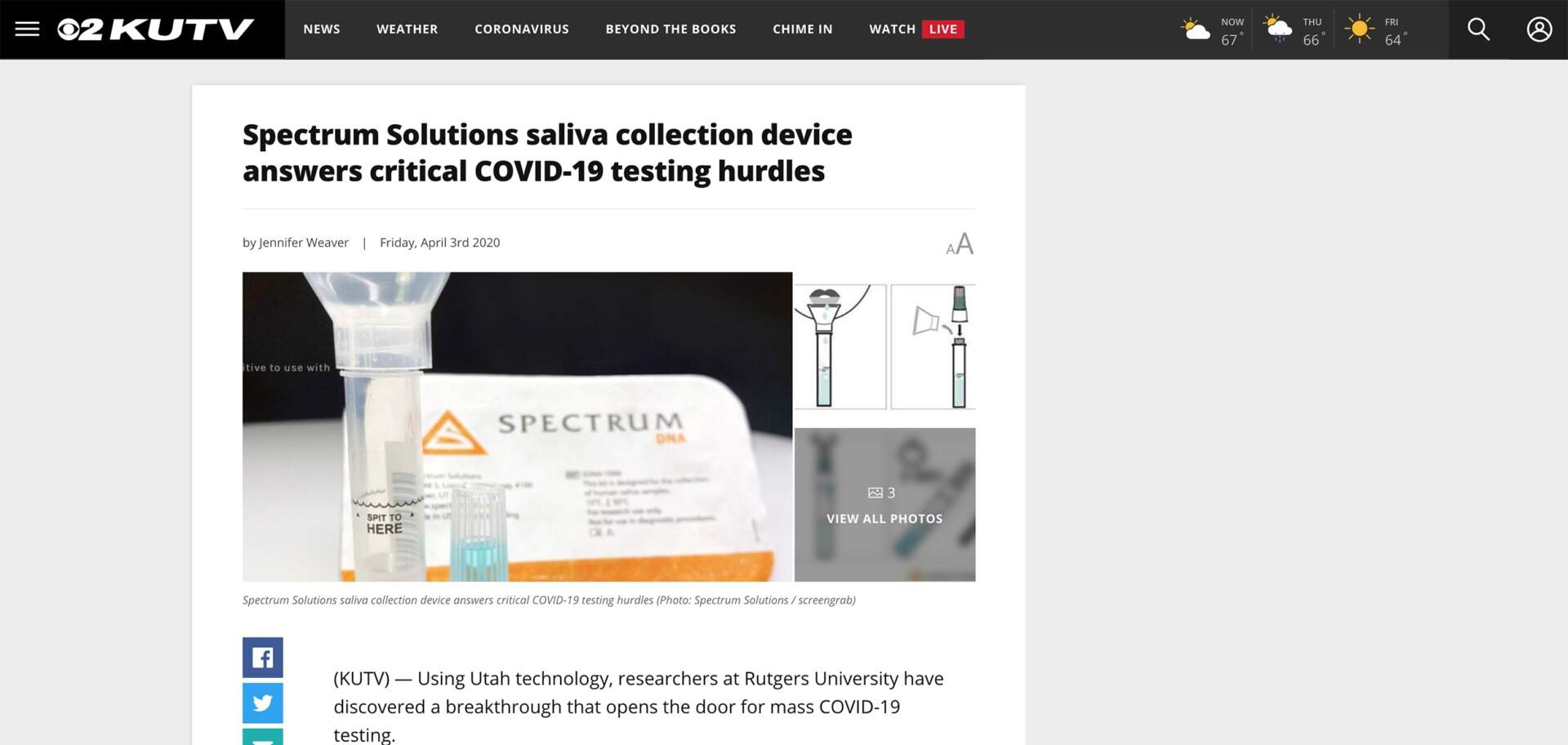
Using Utah technology, researchers at Rutgers University have discovered a breakthrough that opens the door for mass COVID-19 testing.
(KUTV) — Using Utah technology, researchers at Rutgers University have discovered a breakthrough that opens the door for mass COVID-19 testing. Spectrum DNA’s SDNA-1000 Whole Saliva DNA Collection Device is being used by researchers at RUCDR Infinite Biologics at Rutgers University and successfully validated that saliva is an equally viable source for COVID-19 detection as other biomaterials, supplanting the need for scarce testing supplies.
According to a press release, saliva testing is a new development that, under a medical provider’s direction, allows for simple self-administered sample collection that even those in quarantine or self-isolation can use. This same device has been proven effective by millions of people for consumer DNA ancestry-type tests.
According to the researchers, its unique properties including the blue preservation solution excellently preserved the COVID-19 viral RNA for testing while killing the active virus preventing its spread to medical and clinical staff.
Bill Phillips, Spectrum’s Chief Operating Officer, said in a prepared statement:
This is it. This is what makes America so great. Challenge and adversity throw up roadblocks and we partner together to discover, and deliver, not only an answer to the supply shortage for sample collection but uncover a solution that is actually better than what we were using in the first place.
RUCDR is currently testing for COVID-19 using the Spectrum DNA SDNA-1000 whole saliva collection device leveraging two easily accessible COVID-19 detection assays under EUA from the FDA.
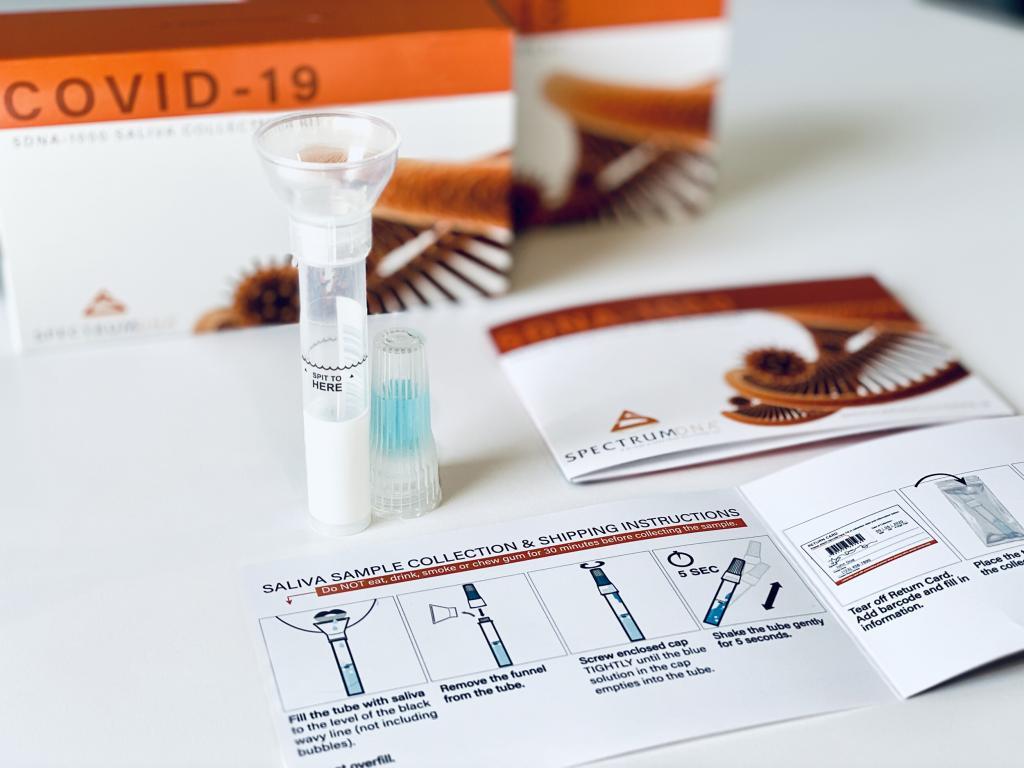
© Spectrum Solutions™ Saliva Collection Device | Photo Credit: Leslie Titus Bryant
Using Utah technology, researchers at Rutgers University have discovered a breakthrough that opens the door for mass COVID-19 testing.
(KUTV) — Using Utah technology, researchers at Rutgers University have discovered a breakthrough that opens the door for mass COVID-19 testing. Spectrum DNA’s SDNA-1000 Whole Saliva DNA Collection Device is being used by researchers at RUCDR Infinite Biologics at Rutgers University and successfully validated that saliva is an equally viable source for COVID-19 detection as other biomaterials, supplanting the need for scarce testing supplies.
According to a press release, saliva testing is a new development that, under a medical provider’s direction, allows for simple self-administered sample collection that even those in quarantine or self-isolation can use. This same device has been proven effective by millions of people for consumer DNA ancestry-type tests.
According to the researchers, its unique properties including the blue preservation solution excellently preserved the COVID-19 viral RNA for testing while killing the active virus preventing its spread to medical and clinical staff.
Bill Phillips, Spectrum’s Chief Operating Officer, said in a prepared statement:
This is it. This is what makes America so great. Challenge and adversity throw up roadblocks and we partner together to discover, and deliver, not only an answer to the supply shortage for sample collection but uncover a solution that is actually better than what we were using in the first place.
RUCDR is currently testing for COVID-19 using the Spectrum DNA SDNA-1000 whole saliva collection device leveraging two easily accessible COVID-19 detection assays under EUA from the FDA.

© Spectrum Solutions™ Saliva Collection Device | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.