ABC NEWS:
Scientist behind saliva test breakthrough sees bridge to nationwide coronavirus screening
By Matthew Mosk and Lucien Bruggeman
Published: April 16, 2020
Published: April 16, 2020
FDA authorizes new saliva COVID-19 test, however, the FDA has yet to authorize in-home testing, but it could be the future.
The Rutgers University scientist who oversaw the development of a saliva test to detect coronavirus said he believes this new way to collect patient samples could serve as a bridge to widespread national testing — modeled off the kits used by familiar commercial genealogical brands like Ancestry.com and 23 and Me.
“It opens up a lot of doors,” Andrew Brooks, the chief operating officer and director of technology development at the Rutgers lab, told ABC News.
Brooks painted a picture of what a future with large-scale, nationwide testing could look like, and it is very similar to the method commercial genealogy firms use to collect their DNA samples from their customers. As with genealogy tests, he said a testing company could ship a kit to the patient, they can spit into a vial, and then the vial is sent to a lab for analysis.
But unlike a genealogy company’s DNA review, this test would need to be prescribed by a physician, and potentially supervised via telehealth, Brooks said. And the results would need to be reported to the Centers for Disease Control and Prevention by a physician.
“It will be a different overall experience because we’re making a medical diagnosis here,” Brooks said, “but the process is the same.” “And for people that don’t have cars, that don’t have access, that are on quarantine. It makes it more broadly available,” Brooks said. “We’re hoping very soon … that may be very well possible.”
Though the FDA granted emergency authorization for the saliva tests, and they’re currently being deployed in several health care facilities in New Jersey, there remains some question as to whether the levels of virus in the saliva would be high enough to be reliably detected, according to ABC News medical contributor Dr. Mark Abdelmalek. Experts in microbiology told ABC News it is plausible that the presence of virus in saliva would theoretically come from the nasal secretions that produce results for a nasal swab.
Brooks said he’s confident in the science, and he has a high profile supporter in the White House. President Donald Trump praised the saliva test method Tuesday as “innovation under pressure.”
Brooks has another good reason to consider the potential of easy-to-use DNA collection kits as a way to scale up coronavirus testing. He is also chief scientist for Spectrum DNA, the Utah-based company that already supplies those kits to Ancestry.com and developed the special preservative that has enabled customers to spit in a tube and mail their saliva samples to the company’s labs for DNA analysis.
The same kinds of tubes can also be used to preserve the crucial sample of RNA needed to determine if a COVID-19 patient has been exposed to the virus, a development a spokesperson for Spectrum called an “incredible breakthrough.”
It would be “amazing” if private companies developed and widely distributed a saliva home-test, said Leslie Bryant, the Spectrum spokesperson.
“I mean think about the volume – talk about serving as many people as possible,” Bryant said.
Large-scale testing is widely viewed as a key element of any plan to re-open the country. Understanding who has, or has had, the disease will allow public health officials to identify which U.S. communities can relax shelter-in-place measures and get some Americans back to work — assuming potential looming privacy issues are settled. And industry experts said scalability is just one of several benefits to an authorized at-home saliva test.
“The convenience, the sheer number of people you can get to – we’re all sheltering in place, you can’t leave, it’s better that you don’t leave,” said Dr. Ruth Ann Crystal, a Stanford-trained obstetrician who works closely with multiple health care technology startups. “At-home testing would be amazing.”
“It completely mitigates the risk of our health care professionals getting infected,” Brooks added.
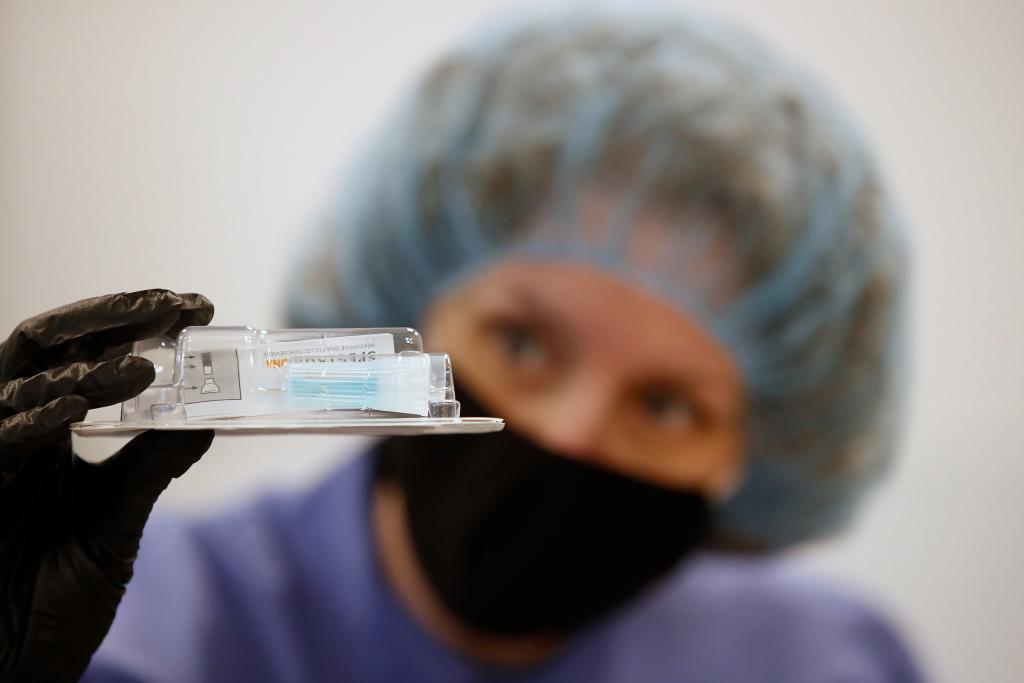
© Spectrum Solutions™ Bloomberg News/George Frey
FDA authorizes new saliva COVID-19 test, however, the FDA has yet to authorize in-home testing, but it could be the future.
The Rutgers University scientist who oversaw the development of a saliva test to detect coronavirus said he believes this new way to collect patient samples could serve as a bridge to widespread national testing — modeled off the kits used by familiar commercial genealogical brands like Ancestry.com and 23 and Me.
“It opens up a lot of doors,” Andrew Brooks, the chief operating officer and director of technology development at the Rutgers lab, told ABC News.
Brooks painted a picture of what a future with large-scale, nationwide testing could look like, and it is very similar to the method commercial genealogy firms use to collect their DNA samples from their customers. As with genealogy tests, he said a testing company could ship a kit to the patient, they can spit into a vial, and then the vial is sent to a lab for analysis.
But unlike a genealogy company’s DNA review, this test would need to be prescribed by a physician, and potentially supervised via telehealth, Brooks said. And the results would need to be reported to the Centers for Disease Control and Prevention by a physician.
“It will be a different overall experience because we’re making a medical diagnosis here,” Brooks said, “but the process is the same.” “And for people that don’t have cars, that don’t have access, that are on quarantine. It makes it more broadly available,” Brooks said. “We’re hoping very soon … that may be very well possible.”
Though the FDA granted emergency authorization for the saliva tests, and they’re currently being deployed in several health care facilities in New Jersey, there remains some question as to whether the levels of virus in the saliva would be high enough to be reliably detected, according to ABC News medical contributor Dr. Mark Abdelmalek. Experts in microbiology told ABC News it is plausible that the presence of virus in saliva would theoretically come from the nasal secretions that produce results for a nasal swab.
Brooks said he’s confident in the science, and he has a high profile supporter in the White House. President Donald Trump praised the saliva test method Tuesday as “innovation under pressure.”
Brooks has another good reason to consider the potential of easy-to-use DNA collection kits as a way to scale up coronavirus testing. He is also chief scientist for Spectrum DNA, the Utah-based company that already supplies those kits to Ancestry.com and developed the special preservative that has enabled customers to spit in a tube and mail their saliva samples to the company’s labs for DNA analysis.
The same kinds of tubes can also be used to preserve the crucial sample of RNA needed to determine if a COVID-19 patient has been exposed to the virus, a development a spokesperson for Spectrum called an “incredible breakthrough.”
It would be “amazing” if private companies developed and widely distributed a saliva home-test, said Leslie Bryant, the Spectrum spokesperson.
“I mean think about the volume – talk about serving as many people as possible,” Bryant said.
Large-scale testing is widely viewed as a key element of any plan to re-open the country. Understanding who has, or has had, the disease will allow public health officials to identify which U.S. communities can relax shelter-in-place measures and get some Americans back to work — assuming potential looming privacy issues are settled. And industry experts said scalability is just one of several benefits to an authorized at-home saliva test.
“The convenience, the sheer number of people you can get to – we’re all sheltering in place, you can’t leave, it’s better that you don’t leave,” said Dr. Ruth Ann Crystal, a Stanford-trained obstetrician who works closely with multiple health care technology startups. “At-home testing would be amazing.”
“It completely mitigates the risk of our health care professionals getting infected,” Brooks added.

© Spectrum Solutions™ Bloomberg News/George Frey
Can genealogy firms help pull it off?
Both Ancestry.com and 23 and Me have, to varying degrees, considered the potential to leverage their presence and infrastructure in the saliva testing business to develop a viable test for the coronavirus.
Dr. Catherine Ball, the chief scientific officer at Ancestry.com, said the company has already shared its test kits with several independent labs across the country.
“[The labs] are in the process of evaluating whether or not our device and our stabilization fluid supports COVID testing,” Ball said. “If it works, it works. We are hopeful that we can make a positive impact if it works.”
A spokesperson for 23 and Me told ABC News that the company “has explored bringing saliva diagnostic tests to market to help with the COVID-19 pandemic response,” but cited several potential roadblocks in doing so any time soon.
“Our collection kit is designed to preserve DNA and, unfortunately, does not have a buffer that preserves viral RNA (which is much more delicate),” the spokesperson said. “We have explored steps to address this, but it is a significant change to our supply chain during a time where many suppliers are necessarily operating at minimal staff.”

Both Ancestry.com and 23 and Me have, to varying degrees, considered the potential to leverage their presence and infrastructure in the saliva testing business to develop a viable test for the coronavirus.
Dr. Catherine Ball, the chief scientific officer at Ancestry.com, said the company has already shared its test kits with several independent labs across the country.
“[The labs] are in the process of evaluating whether or not our device and our stabilization fluid supports COVID testing,” Ball said. “If it works, it works. We are hopeful that we can make a positive impact if it works.”
A spokesperson for 23 and Me told ABC News that the company “has explored bringing saliva diagnostic tests to market to help with the COVID-19 pandemic response,” but cited several potential roadblocks in doing so any time soon.
“Our collection kit is designed to preserve DNA and, unfortunately, does not have a buffer that preserves viral RNA (which is much more delicate),” the spokesperson said. “We have explored steps to address this, but it is a significant change to our supply chain during a time where many suppliers are necessarily operating at minimal staff.”

Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.