ABC NEWS:
WASHINGTON POST
HEALTH 202: The newest way to test for coronavirus: Spitting in a tube
HEALTH 202: The newest way to test for coronavirus: Spitting in a tube
By Matthew Mosk and Jay Bhatt
Published: April 15, 2020
Published: April 15, 2020
Scientist behind saliva test breakthrough sees bridge to nationwide coronavirus screening
In a Rose Garden briefing, Tuesday President Donald Trump touted a potential new way of testing for the novel coronavirus could be safer for suspected coronavirus patients and health care workers and help alleviate the backlog in hard-hit cities — spit.
The Food and Drug Administration gave emergency authorization Friday for a testing method developed by a Rutgers University lab that, instead of relying on swabs that reach deep into the nasal cavity, is designed to check for evidence of the virus in a patient’s saliva.
“I call it innovation under pressure,” Trump said. “The tests can be self-administered by patients in health care settings, which will reduce exposure for medical workers and save personal protective equipment.”
Rutgers said the saliva tests are already being made available to the large New Jersey health care system RWJBarnabas and at a number of New Jersey hospitals and facilities. A drive-thru testing facility utilizing this new method is also planned this week in Edison, New Jersey.
Though the tests are only now ramping up, already several companies have begun working on manufacturing and selling versions of them, including the start-up Vault Health, a New York-based men’s health company.
“This is not an at-home test,” Hahn said of the government-authorized method. “This still has to be performed with a provider, but it does provide great advantages moving forward and expands the opportunities for testing.”
Abdelmalek said concerns about self-administered tests stem in part from the worry that the patient could make an error that leads to an incorrect outcome — and waste valuable testing resources in the process.
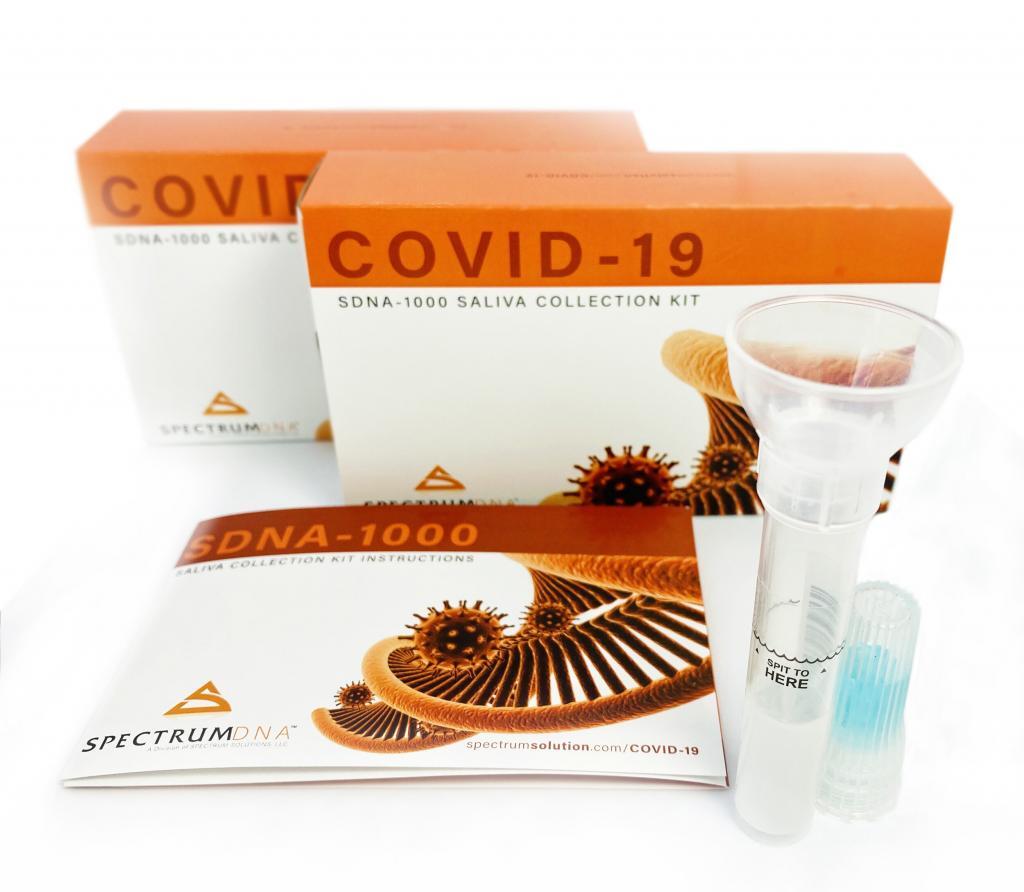
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Scientist behind saliva test breakthrough sees bridge to nationwide coronavirus screening
In a Rose Garden briefing, Tuesday President Donald Trump touted a potential new way of testing for the novel coronavirus could be safer for suspected coronavirus patients and health care workers and help alleviate the backlog in hard-hit cities — spit.
The Food and Drug Administration gave emergency authorization Friday for a testing method developed by a Rutgers University lab that, instead of relying on swabs that reach deep into the nasal cavity, is designed to check for evidence of the virus in a patient’s saliva.
“I call it innovation under pressure,” Trump said. “The tests can be self-administered by patients in health care settings, which will reduce exposure for medical workers and save personal protective equipment.”
Rutgers said the saliva tests are already being made available to the large New Jersey health care system RWJBarnabas and at a number of New Jersey hospitals and facilities. A drive-thru testing facility utilizing this new method is also planned this week in Edison, New Jersey.
Though the tests are only now ramping up, already several companies have begun working on manufacturing and selling versions of them, including the start-up Vault Health, a New York-based men’s health company.
“This is not an at-home test,” Hahn said of the government-authorized method. “This still has to be performed with a provider, but it does provide great advantages moving forward and expands the opportunities for testing.”
Abdelmalek said concerns about self-administered tests stem in part from the worry that the patient could make an error that leads to an incorrect outcome — and waste valuable testing resources in the process.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.


