BIOWORLD:
THE NEW YORK TIMES: Just Spit and Wait – New Coronavirus Test Offers Advantage
By Meg Bryant
Published: May 11, 2020
Published: May 11, 2020
The U.S. FDA has expanded the emergency use authorization (EUA) for Rutgers University’s saliva-based COVID-19 test to include at-home use of Spectrum Solutions LLC’s SDNA-1000 whole saliva collection device, a development that underscores the need for wider testing as localities across the country emerge from lockdown and restart their economies.
This is the first at-home option for saliva collection; the action comes just two weeks after the FDA authorized Laboratory Corp. of America’s polymerase chain reaction test for SARS-CoV-2, the virus that causes COVID-19, to include the company’s Pixel self-collection kit for nasal samples.
In announcing the decision, FDA Commissioner Stephen Hahn said enabling people to self-collect samples for the Rutgers Clinical Genomics Laboratory’s test “provides an additional option for the easy, safe, and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site.”
Spectrum CEO Stephen Fanning said the move highlights the agency’s recognition that “current methods of obtaining biosamples for COVID-19 testing were limited in scope and scale due to supply shortages[,] and the current testing methods were also constantly putting health care workers at risk of exposure. We’ve developed a better, more innovative solution.”
As of May 10, more than 8.3 million people in the U.S. have been tested for COVID-19, but Anthony Fauci, director of the National Institute for Allergy and Infectious Diseases and a member of the White House Coronavirus Task Force, has said the current rate of 1.5 million to 2 million tests per week needs to double by the end of May.
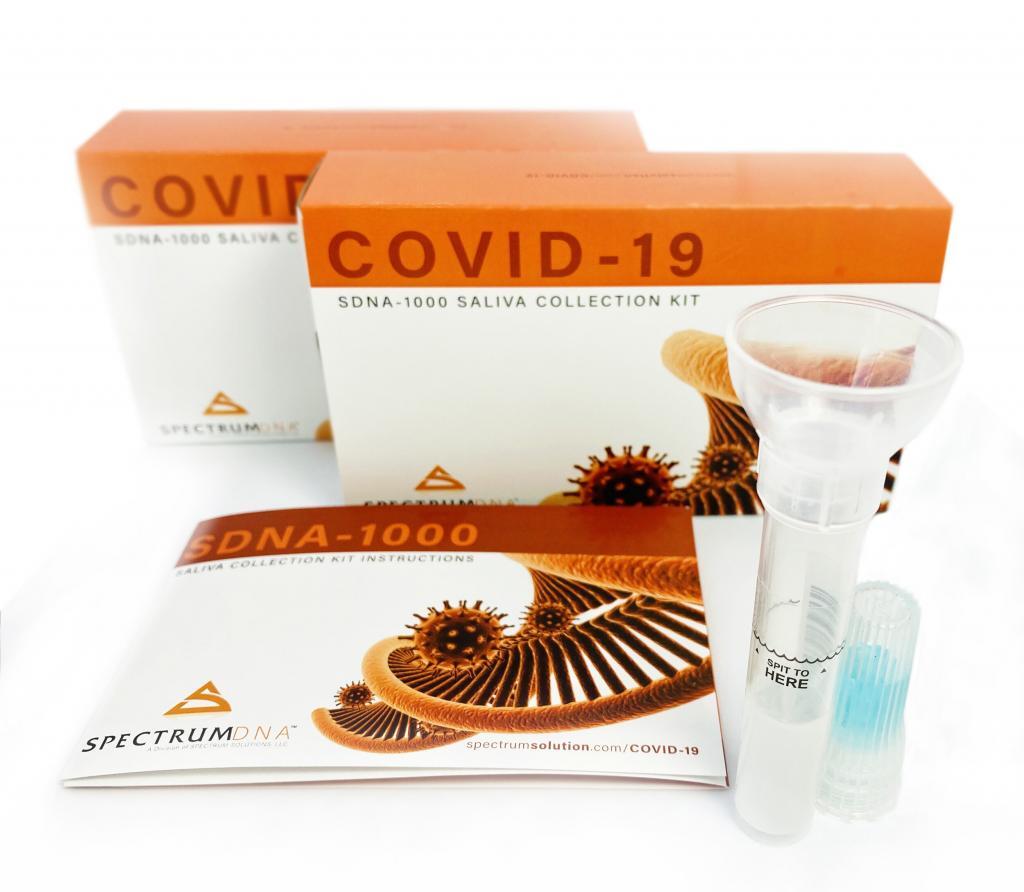
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
The U.S. FDA has expanded the emergency use authorization (EUA) for Rutgers University’s saliva-based COVID-19 test to include at-home use of Spectrum Solutions LLC’s SDNA-1000 whole saliva collection device, a development that underscores the need for wider testing as localities across the country emerge from lockdown and restart their economies.
This is the first at-home option for saliva collection; the action comes just two weeks after the FDA authorized Laboratory Corp. of America’s polymerase chain reaction test for SARS-CoV-2, the virus that causes COVID-19, to include the company’s Pixel self-collection kit for nasal samples.
In announcing the decision, FDA Commissioner Stephen Hahn said enabling people to self-collect samples for the Rutgers Clinical Genomics Laboratory’s test “provides an additional option for the easy, safe, and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site.”
Spectrum CEO Stephen Fanning said the move highlights the agency’s recognition that “current methods of obtaining biosamples for COVID-19 testing were limited in scope and scale due to supply shortages[,] and the current testing methods were also constantly putting health care workers at risk of exposure. We’ve developed a better, more innovative solution.”
As of May 10, more than 8.3 million people in the U.S. have been tested for COVID-19, but Anthony Fauci, director of the National Institute for Allergy and Infectious Diseases and a member of the White House Coronavirus Task Force, has said the current rate of 1.5 million to 2 million tests per week needs to double by the end of May.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
We’ve developed a better, more innovative solution.
-Stephen Fanning, CEO Spectrum Solutions
Robust safety testing and validation
The SNDA-1000 test kit was developed by RUCDR Infinite Biologics, a Rutgers laboratory, in collaboration with Salt Lake City-based Spectrum and Accurate Diagnostic Labs Inc., of Plainfield, N.J., and validated as a viable alternative to nasal and oral swabs for diagnosing COVID-19. In early April, the FDA authorized it for use with Rutgers’ molecular-based laboratory-developed test for the detection of nucleic acid from SARS-CoV-2, but patients needed to go to a hospital or testing site to provide saliva. Available by prescription, it is currently the only FDA-authorized COVID-19 diagnostic that uses a patient’s spit to test for SARS-CoV-2.
For the new at-home self-collection authorization, the FDA’s main focus was ensuring that the collection kit could deliver the required quality samples. The device is bacteriostatic and uses a preservation solution that stabilizes viral samples with the saliva, reducing exposure risks to those performing the tests.
“The SDNA-1000 easily displayed its might in shipping simulations at extreme temperatures for up to about 10 days, proving the sample stability and integrity,” Leslie Bryant, Spectrum’s director of marketing and brand, told BioWorld. “In terms of safety concerns, they wanted to understand if, for some reason, someone decided to drink the blue solution and saliva mixture once they put in in the tube, [that person] would be okay.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
The SNDA-1000 test kit was developed by RUCDR Infinite Biologics, a Rutgers laboratory, in collaboration with Salt Lake City-based Spectrum and Accurate Diagnostic Labs Inc., of Plainfield, N.J., and validated as a viable alternative to nasal and oral swabs for diagnosing COVID-19. In early April, the FDA authorized it for use with Rutgers’ molecular-based laboratory-developed test for the detection of nucleic acid from SARS-CoV-2, but patients needed to go to a hospital or testing site to provide saliva. Available by prescription, it is currently the only FDA-authorized COVID-19 diagnostic that uses a patient’s spit to test for SARS-CoV-2.
For the new at-home self-collection authorization, the FDA’s main focus was ensuring that the collection kit could deliver the required quality samples. The device is bacteriostatic and uses a preservation solution that stabilizes viral samples with the saliva, reducing exposure risks to those performing the tests.
“The SDNA-1000 easily displayed its might in shipping simulations at extreme temperatures for up to about 10 days, proving the sample stability and integrity,” Leslie Bryant, Spectrum’s director of marketing and brand, told BioWorld. “In terms of safety concerns, they wanted to understand if, for some reason, someone decided to drink the blue solution and saliva mixture once they put in in the tube, [that person] would be okay.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Designed to avoid user error
Under the expanded EUA, Individuals wishing to purchase the test must meet with a doctor – either in person or via a telemedicine visit – to get a prescription and arrange for the test to be delivered to their home. Once the sample is collected, it is returned in a sealed package to Rutgers Clinical Genomic Laboratory in New Jersey for testing, and results are reported to the patient’s doctor. Turnaround time is 48 hours.
“Spectrum engineered its SDNA-1000 saliva collection device to eliminate common user collection errors in a universally intuitive, three-step process which provides visual confirmation when successfully completed,” said Jeremy Johnson, Spectrum R&D engineer. “We not only engineered this device to make self-collection intuitive, we also added a preservation chemistry that has proven itself to be the catalyst needed to deliver to labs the quality samples required for accurate COVID-19 detection.”
Bryant said the company is operating around the clock to scale production of SDNA-1000 collection kits from more than 1 million per month currently to 3 million per month in June and 15 million kits a month by the end of the year.

© Spectrum Solutions™ COVID-19 SDNA-1000 Saliva Collection Testing Kit | Bloomberg News/George Frey
Under the expanded EUA, Individuals wishing to purchase the test must meet with a doctor – either in person or via a telemedicine visit – to get a prescription and arrange for the test to be delivered to their home. Once the sample is collected, it is returned in a sealed package to Rutgers Clinical Genomic Laboratory in New Jersey for testing, and results are reported to the patient’s doctor. Turnaround time is 48 hours.
“Spectrum engineered its SDNA-1000 saliva collection device to eliminate common user collection errors in a universally intuitive, three-step process which provides visual confirmation when successfully completed,” said Jeremy Johnson, Spectrum R&D engineer. “We not only engineered this device to make self-collection intuitive, we also added a preservation chemistry that has proven itself to be the catalyst needed to deliver to labs the quality samples required for accurate COVID-19 detection.”
Bryant said the company is operating around the clock to scale production of SDNA-1000 collection kits from more than 1 million per month currently to 3 million per month in June and 15 million kits a month by the end of the year.

© Spectrum Solutions™ COVID-19 SDNA-1000 Saliva Collection Testing Kit | Bloomberg News/George Frey
Testing in Europe and Asia
The FDA has authorized a host of COVID-19 tests. Many are available outside the U.S. as well, via CE mark or other country approvals. For example, last week, Switzerland-based Roche Holding AG announced the receipt of both an EUA and CE-IVD certification for its Elecsys Anti-SARS-CoV-2 serology test to detect antibodies in people previously exposed to the virus.
Several French startups are active in the space. For its part, Biosynex SA has announced plans to mass-produce a rapid immunochromatographic serological test to detect COVID-19 antibodies in a drop of blood, while Ng Biotech SAAS has rolled out a rapid test for SARS-CoV-2.
Meanwhile, in China, where the coronavirus originated, Innovita Biological Technology Co. Ltd. has developed a test to detect IgG and IgM antibodies in 15 minutes.
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.