BIOWORLD:
FDA Stenzel: EUA for COVID-19 at-home collection sample kit will happen ‘very soon’
By Mark McCarty
Published: April 15, 2020
Published: April 15, 2020
The test, developed by a Rutgers lab, could be a safer and faster alternative using saliva collection.
An April 15 U.S. FDA stakeholder call revisited several themes of interest in connection with diagnostics for the COVID-19 pandemic. However, Tim Stenzel, director of the agency’s Office of In Vitro Diagnostics and Radiological Health, said that while the agency has not yet authorized a home sample collection kit, “we do think it’s going to happen very soon.”
The agency routinely has posted new developments related to diagnostics for the SARS-CoV-2 virus, and Stenzel noted that among the new diagnostic entries to the emergency use authorization (EUA) is the April 14 authorization for Chembio Diagnostic Systems Inc., of Medford, N.Y. This EUA is a relatively rare authorization for serology testing, which in this instance can be conducted with either fingerstick or whole blood. Stenzel noted that the agency has issued a EUA for a saliva test from Rutgers University using a saliva collection kit devised by Spectrum Solutions LLC, of Salt Lake City.
Stenzel added that the agency is looking forward to authorizing the first home sample collection test, but that it had not done so as of early afternoon April 15. A home collection kit is not the only advancement the agency hopes to see in the coming days and weeks. He said there is also no authorization for a home test, but that the FDA is actively working with “a number of developers” on such a test for the virus. The saliva test “remains a very interesting sample type” for the FDA, Stenzel said, although the agency is concerned about sample stability in connection this approach.
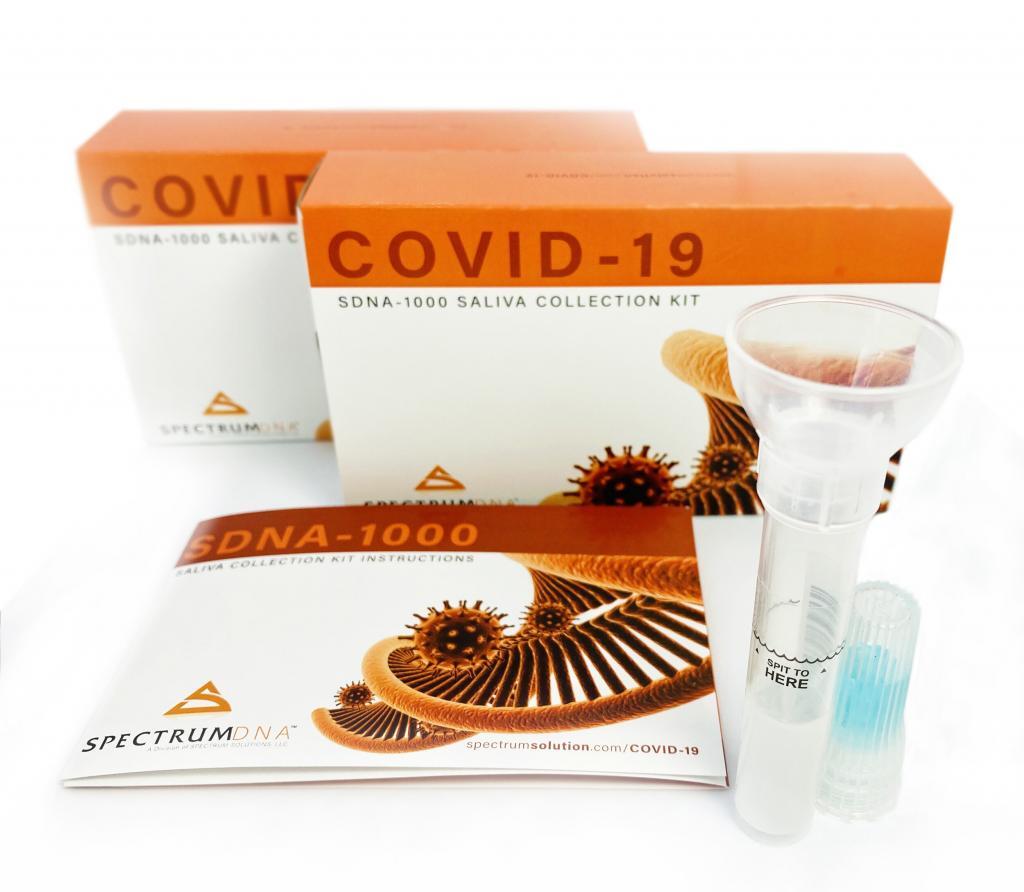
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
The test, developed by a Rutgers lab, could be a safer and faster alternative using saliva collection.
An April 15 U.S. FDA stakeholder call revisited several themes of interest in connection with diagnostics for the COVID-19 pandemic. However, Tim Stenzel, director of the agency’s Office of In Vitro Diagnostics and Radiological Health, said that while the agency has not yet authorized a home sample collection kit, “we do think it’s going to happen very soon.”
The agency routinely has posted new developments related to diagnostics for the SARS-CoV-2 virus, and Stenzel noted that among the new diagnostic entries to the emergency use authorization (EUA) is the April 14 authorization for Chembio Diagnostic Systems Inc., of Medford, N.Y. This EUA is a relatively rare authorization for serology testing, which in this instance can be conducted with either fingerstick or whole blood. Stenzel noted that the agency has issued a EUA for a saliva test from Rutgers University using a saliva collection kit devised by Spectrum Solutions LLC, of Salt Lake City.
Stenzel added that the agency is looking forward to authorizing the first home sample collection test, but that it had not done so as of early afternoon April 15. A home collection kit is not the only advancement the agency hopes to see in the coming days and weeks. He said there is also no authorization for a home test, but that the FDA is actively working with “a number of developers” on such a test for the virus. The saliva test “remains a very interesting sample type” for the FDA, Stenzel said, although the agency is concerned about sample stability in connection this approach.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
False positives still a concern for serology
“We continue to see problematic claims” in connection with serology tests, Stenzel said, which are listed under policy D in the agency’s FAQ series. Under this policy, a serology test can be conducted only in a clinical lab only that is certified under CLIA to perform high-complexity tests. Stenzel said false positives are a significant risk that the FDA remains concerned about, and the agency will in the coming days and weeks seek to highlight those concerns, even in connection with highly specific tests deployed in very low-prevalence areas. He said to stay tuned on this and several other considerations.
The FDA has spent a fair amount of time on some questions surrounding the use of additive manufacturing for nasopharyngeal swabs. Stenzel said the agency has seen some creative ideas but suggested that sponsors contact the agency for advice.
State governments may authorize lab-developed tests (LDTs), although the FDA’s policy is to limit such a practice for entities certified under CLIA for high-complexity tests. New York, for example, is known for state regulation of clinical labs. However, FDA policy is not prescriptive as to how the states will determine the LDT authorization process. Stenzel said the agency prefers that any associated collection devices be vetted by the agency, however.
The FDA can withdraw the EUA for a specific test if it is not performing, but Stenzel said the agency would first contact the developer to find a useful path for sustaining test availability. This was done in connection with the ID Now test by Abbott, and Stenzel said the emphasis will be to develop an understanding of the performance issues before pulling the plug on a EUA.
Stenzel said he does not foresee the COVID-19 EUA mechanism disappearing anytime soon, adding that it may be in place for another year. This hinges on the expectation that the virus will resurface in the coming flu season and that “herd immunity” will not have been accomplished by then, particularly given the low likelihood that a vaccine will be available at any point in 2020. He advised that if a test developer files a conventional premarket regulatory filing for a EUA test, the statute allows the agency to declare that any similar devices no longer fall under the EAU mechanism. However, he said the FDA would undertake such a move “only very carefully” due to the need to ensure test availability.

© Spectrum Solutions™ | Bloomberg News/George Frey
“We continue to see problematic claims” in connection with serology tests, Stenzel said, which are listed under policy D in the agency’s FAQ series. Under this policy, a serology test can be conducted only in a clinical lab only that is certified under CLIA to perform high-complexity tests. Stenzel said false positives are a significant risk that the FDA remains concerned about, and the agency will in the coming days and weeks seek to highlight those concerns, even in connection with highly specific tests deployed in very low-prevalence areas. He said to stay tuned on this and several other considerations.
The FDA has spent a fair amount of time on some questions surrounding the use of additive manufacturing for nasopharyngeal swabs. Stenzel said the agency has seen some creative ideas but suggested that sponsors contact the agency for advice.
State governments may authorize lab-developed tests (LDTs), although the FDA’s policy is to limit such a practice for entities certified under CLIA for high-complexity tests. New York, for example, is known for state regulation of clinical labs. However, FDA policy is not prescriptive as to how the states will determine the LDT authorization process. Stenzel said the agency prefers that any associated collection devices be vetted by the agency, however.
The FDA can withdraw the EUA for a specific test if it is not performing, but Stenzel said the agency would first contact the developer to find a useful path for sustaining test availability. This was done in connection with the ID Now test by Abbott, and Stenzel said the emphasis will be to develop an understanding of the performance issues before pulling the plug on a EUA.
Stenzel said he does not foresee the COVID-19 EUA mechanism disappearing anytime soon, adding that it may be in place for another year. This hinges on the expectation that the virus will resurface in the coming flu season and that “herd immunity” will not have been accomplished by then, particularly given the low likelihood that a vaccine will be available at any point in 2020. He advised that if a test developer files a conventional premarket regulatory filing for a EUA test, the statute allows the agency to declare that any similar devices no longer fall under the EAU mechanism. However, he said the FDA would undertake such a move “only very carefully” due to the need to ensure test availability.

© Spectrum Solutions™ | Bloomberg News/George Frey
Policy on 3D swab manufacture in the works
The FDA signed a memorandum of understanding with the NIH and the Department of Veteran’s Health regarding additive manufacturing for a variety of items, including personal protective equipment. Stenzel said the agency is considering a policy regarding any swabs produced as a result of 3D printing that would stipulate whether such items must be manufactured under conventional regulatory regimes. This policy, which is not yet in the public domain, likely would mandate that such items be checked for biocompatibility as well.
The FDA has a series of templates for EUA applications, including diagnostic testing, but Stenzel said the agency does not yet have a template for rapid testing via lateral flow technology. The FDA is working on a EUA template for serology tests, although further templates will become available as the agency is able to develop them.
© Spectrum Solutions™ | Bloomberg News/George Frey
The FDA signed a memorandum of understanding with the NIH and the Department of Veteran’s Health regarding additive manufacturing for a variety of items, including personal protective equipment. Stenzel said the agency is considering a policy regarding any swabs produced as a result of 3D printing that would stipulate whether such items must be manufactured under conventional regulatory regimes. This policy, which is not yet in the public domain, likely would mandate that such items be checked for biocompatibility as well.
The FDA has a series of templates for EUA applications, including diagnostic testing, but Stenzel said the agency does not yet have a template for rapid testing via lateral flow technology. The FDA is working on a EUA template for serology tests, although further templates will become available as the agency is able to develop them.

© Spectrum Solutions™ | Bloomberg News/George Frey
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
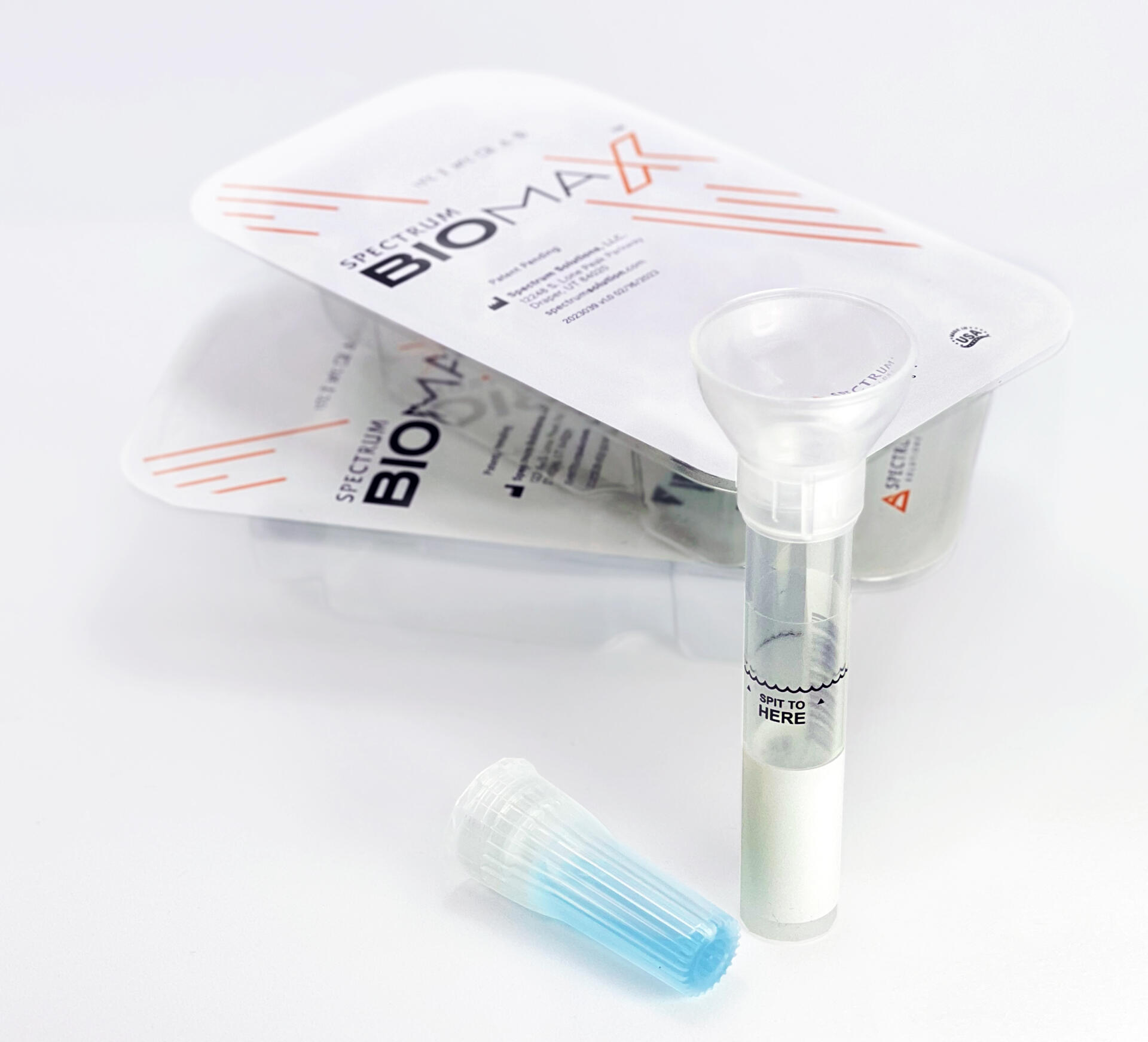
Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.

