BIOWORLD:
First saliva test gets EUA from FDA to detect COVID-19
By Stacy Lawrence
Published: April 15, 2020
Published: April 15, 2020
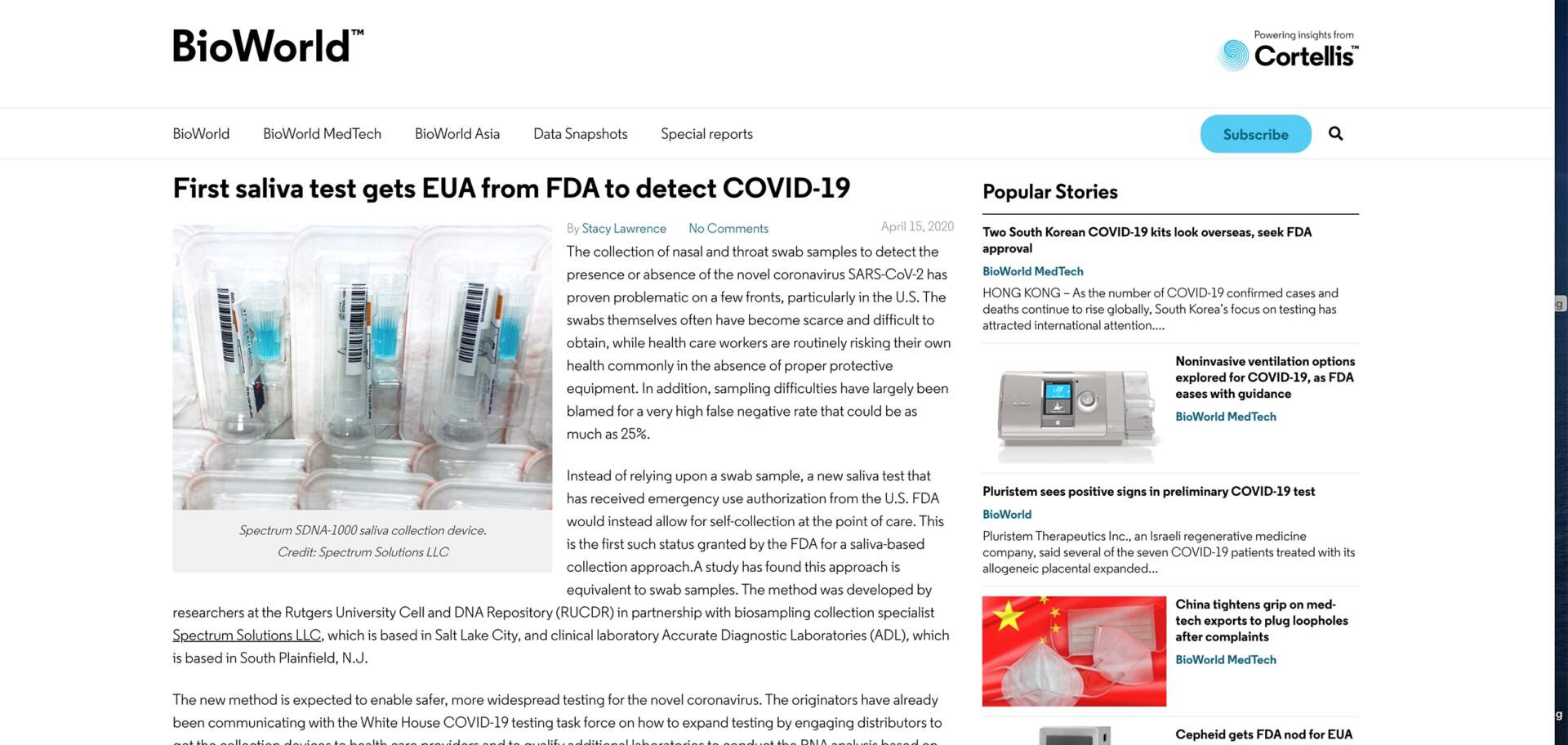
Instead of relying upon a swab sample, a new saliva test that has received emergency use authorization from the U.S. FDA would instead allow for self-collection at the point of care. This is the first such status granted by the FDA for a saliva-based collection approach. A study has found this approach is equivalent to swab samples. The method was developed by researchers at the Rutgers University Cell and DNA Repository (RUCDR) in partnership with biosampling collection specialist Spectrum Solutions LLC, which is based in Salt Lake City, and clinical laboratory Accurate Diagnostic Laboratories (ADL), which is based in South Plainfield, N.J.
The new method is expected to enable safer, more widespread testing for the novel coronavirus. The originators have already been communicating with the White House COVID-19 testing task force on how to expand testing by engaging distributors to get the collection devices to health care providers and to qualify additional laboratories to conduct the RNA analysis based on this type of sample.
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Instead of relying upon a swab sample, a new saliva test that has received emergency use authorization from the U.S. FDA would instead allow for self-collection at the point of care. This is the first such status granted by the FDA for a saliva-based collection approach. A study has found this approach is equivalent to swab samples. The method was developed by researchers at the Rutgers University Cell and DNA Repository (RUCDR) in partnership with biosampling collection specialist Spectrum Solutions LLC, which is based in Salt Lake City, and clinical laboratory Accurate Diagnostic Laboratories (ADL), which is based in South Plainfield, N.J.
The new method is expected to enable safer, more widespread testing for the novel coronavirus. The originators have already been communicating with the White House COVID-19 testing task force on how to expand testing by engaging distributors to get the collection devices to health care providers and to qualify additional laboratories to conduct the RNA analysis based on this type of sample.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Safer sampling
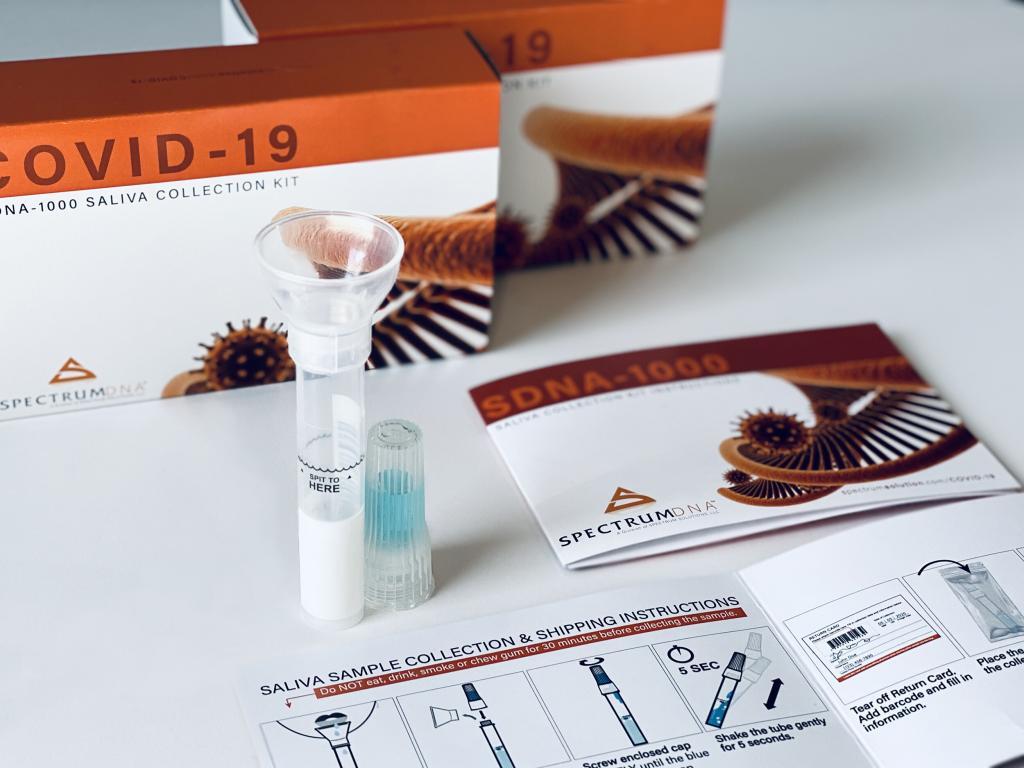
“It doesn’t require a health care worker,” explained Spectrum CEO Stephen Fanning of the saliva collection device to BioWorld. “So, anyone can just be handed it. Right now, with the current swab, the health care worker needs to be there and there’s a chance that he or she could become infected.”
“The way in which we preserve the liquid that’s in it does a couple things,” he continued. “It basically gets rid of the virus, but it leaves the RNA markers. When those markers get there, it’s in a very robust solution so they can tell if, in fact, you have the virus. If the health care worker is opening that tube once it’s sent to the lab, there’s not any way that worker could be contaminated because the virus is dead in the tube. But what we’ve been able to keep are the RNA markers in suspension, and that they can again determine if you have it or not.”
Spectrum can currently produce roughly one million saliva collection devices a month; the company is in the process of acquiring more injection molding machines and within the next two to three weeks aims to triple or even quadruple that initial capacity.
High-volume testing is a crucial component in plans being developed to more safely reopen the U.S. economy and monitor disease prevalence; some approaches call for large-scale, routine testing, while others remain focused on contact tracing and/or would target specific groups such as health care workers or the most vulnerable such as the elderly or chronic disease patients.
A plan from former FDA commissioner Scott Gottlieb, who is a Resident Fellow at the American Enterprise Institute, a conservative think tank, calls for a minimum U.S. capacity of 750,000 tests per week.

©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
“It doesn’t require a health care worker,” explained Spectrum CEO Stephen Fanning of the saliva collection device to BioWorld. “So, anyone can just be handed it. Right now, with the current swab, the health care worker needs to be there and there’s a chance that he or she could become infected.”
“The way in which we preserve the liquid that’s in it does a couple things,” he continued. “It basically gets rid of the virus, but it leaves the RNA markers. When those markers get there, it’s in a very robust solution so they can tell if, in fact, you have the virus. If the health care worker is opening that tube once it’s sent to the lab, there’s not any way that worker could be contaminated because the virus is dead in the tube. But what we’ve been able to keep are the RNA markers in suspension, and that they can again determine if you have it or not.”
Spectrum can currently produce roughly one million saliva collection devices a month; the company is in the process of acquiring more injection molding machines and within the next two to three weeks aims to triple or even quadruple that initial capacity.
High-volume testing is a crucial component in plans being developed to more safely reopen the U.S. economy and monitor disease prevalence; some approaches call for large-scale, routine testing, while others remain focused on contact tracing and/or would target specific groups such as health care workers or the most vulnerable such as the elderly or chronic disease patients.
A plan from former FDA commissioner Scott Gottlieb, who is a Resident Fellow at the American Enterprise Institute, a conservative think tank, calls for a minimum U.S. capacity of 750,000 tests per week.

©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Test Validation
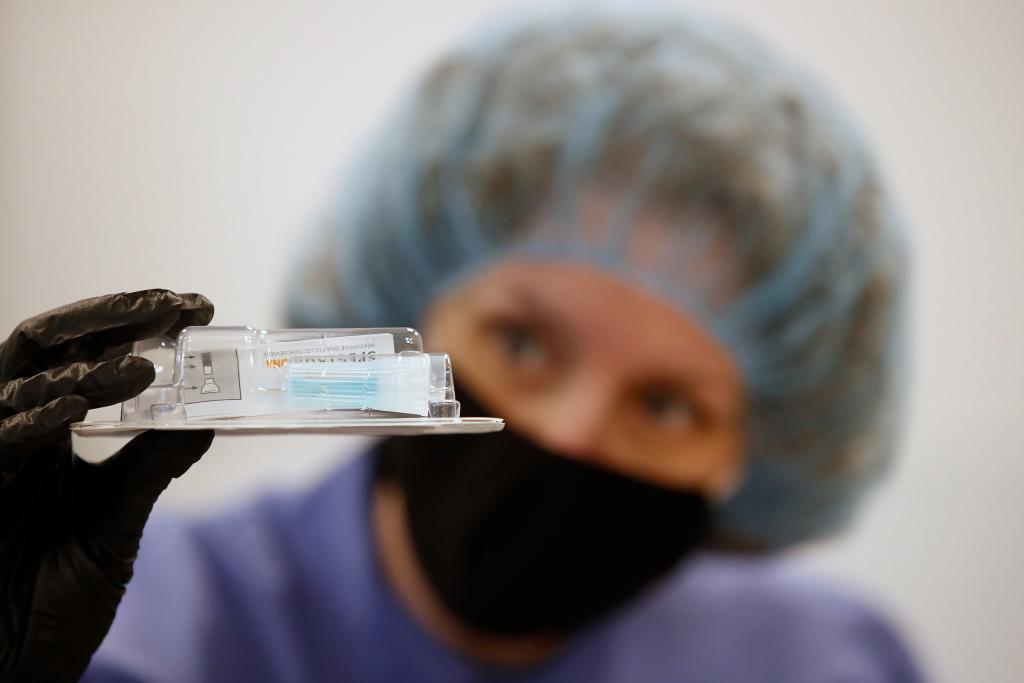
The saliva testing method was validated in a study to confirm saliva could be used in place of nasal pharyngeal or oral pharyngeal swabs for detecting COVID-19 in both symptomatic and asymptomatic patients. The testing found 100% concordance in detecting a patient that had the virus between the methods. It remains unclear thus far if the novel saliva approach could improve upon the inconsistencies in the swab-based sample collection process, which have been widely scrutinized.
Known as the SDNA-1000 Saliva Collection Device, it was used in the study with the Thermo Fisher Taqpath SARS-CoV-2 Assay, which already had an emergency use authorization from the FDA for use with a swab sample that dates back to mid-March. It is a multiplex real-time, PCR qualitative test. The Rutgers lab also used Perkin Elmer nucleic acid extraction.
“The preservation solution was a main focus of the study to ensure that the virus would be inactivated to the point that we would be able to maintain and stabilize the RNA transcripts for sensitive and specific QPCR analysis in order to determine whether a person has the virus,” said Andrew Brooks, chief operating officer at RUCDR Infinite Biologics and Professor of Genetics at Rutgers University. “The device’s intuitive ease-of-use to facilitate minimally supervised or in most cases complete self-collection is a tremendous advance to current COVID-19 sample collection strategies. Together, these benefits will significantly add to expanding access to critical testing needs.”
© Spectrum Solutions™ Bloomberg News/George Frey
The saliva testing method was validated in a study to confirm saliva could be used in place of nasal pharyngeal or oral pharyngeal swabs for detecting COVID-19 in both symptomatic and asymptomatic patients. The testing found 100% concordance in detecting a patient that had the virus between the methods. It remains unclear thus far if the novel saliva approach could improve upon the inconsistencies in the swab-based sample collection process, which have been widely scrutinized.
Known as the SDNA-1000 Saliva Collection Device, it was used in the study with the Thermo Fisher Taqpath SARS-CoV-2 Assay, which already had an emergency use authorization from the FDA for use with a swab sample that dates back to mid-March. It is a multiplex real-time, PCR qualitative test. The Rutgers lab also used Perkin Elmer nucleic acid extraction.
“The preservation solution was a main focus of the study to ensure that the virus would be inactivated to the point that we would be able to maintain and stabilize the RNA transcripts for sensitive and specific QPCR analysis in order to determine whether a person has the virus,” said Andrew Brooks, chief operating officer at RUCDR Infinite Biologics and Professor of Genetics at Rutgers University. “The device’s intuitive ease-of-use to facilitate minimally supervised or in most cases complete self-collection is a tremendous advance to current COVID-19 sample collection strategies. Together, these benefits will significantly add to expanding access to critical testing needs.”

© Spectrum Solutions™ Bloomberg News/George Frey
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.