Breakthrough Saliva Discovery
THE NEW YORK TIMES
Just Spit and Wait – New Coronavirus Test Offers Advantage
Saliva is one of our biggest foes in the COVID-19 pandemic, because of its role in spreading the virus. But it could also prove to be our very best friend too offering a way to diagnose the disease without using invasive nasal swabs.
When COVID-19 testing began in earnest, the most common biosample used to test for the virus was the nasopharyngeal approach, which required a medical professional to conduct a (usually painful) nasal swab on a patient. This, coupled with risk to healthcare workers and a global shortage of swabs for sampling caused researchers around the world to seek viable alternatives.
Rutgers Proves Viability of Saliva for COVID-19 Testing

[Dr. Andrew Brooks, COO at RUCDR]
- Breakthrough Results: There was 100% positive and negative agreement between the results obtained from testing of saliva and those obtained from nasopharyngeal and oropharyngeal swabs. Researchers at Rutgers’s RUCDR Infinite Biologics compared swab-collected biosamples head-to-head against saliva biosamples using the Spectrum DNA whole saliva collection device and its patented blue preservation solution. Saliva not only demonstrated to be a robust source of viral RNA but when preserved with Spectrum’s patented preservation solution it was seen to deliver 100% inactivation of the live virus. Additionally, Spectrum’s saliva collection device provided over 10-days of post-collection stability with no degradation of sample efficacy. Self-collection along with the inactivation of the virus creates the most robust and safest biomaterial collection approach for the detection of COVID-19 infections.
- Researcher Quote: “The impact of this authorization is significant,” said Andrew Brooks, chief operating officer and director of technology development at RUCDR, who also is a professor in the School of Arts and Sciences Department of Genetics at Rutgers University–New Brunswick. “It means we no longer have to put health care professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections. We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as the self-collection of saliva is more quick and scalable than swab collections. All of this combined will have a tremendous impact on testing … across the United States.”
University of Queensland
Is Saliva Friend?Foe?
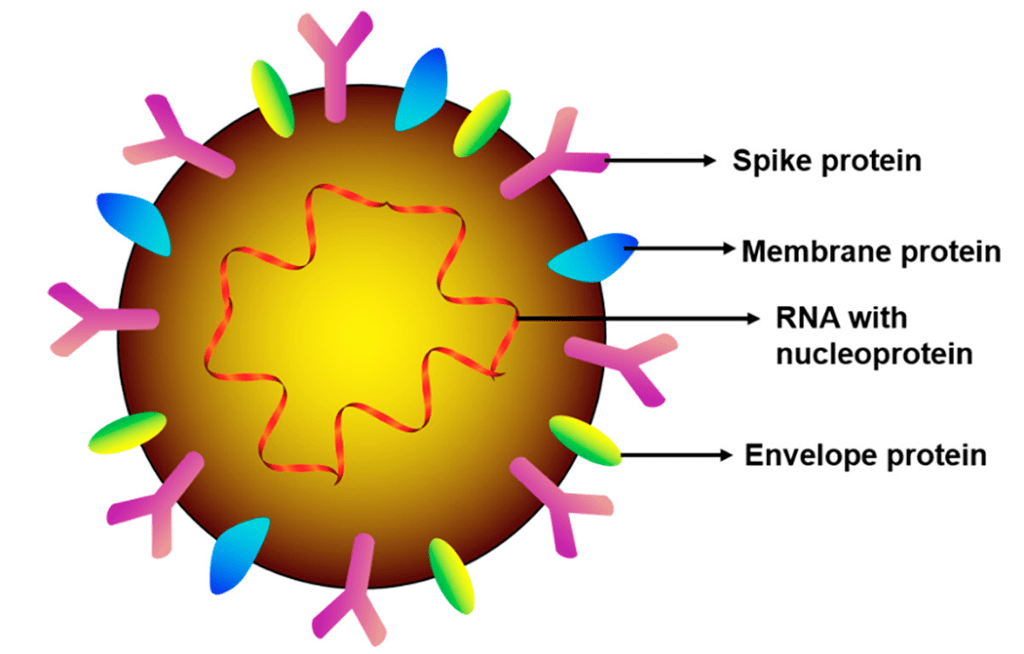 The coronavirus disease 2019 (COVID-19) outbreak, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became a global ongoing pandemic. Timely, accurate, and non-invasive SARS-CoV-2 detection in both symptomatic and asymptomatic patients, as well as the determination of their immune status, will facilitate effective large-scale pandemic control measures to prevent the spread of COVID-19. This review focuses on the role of saliva as both a foe (a common mode of viral transmission via salivary droplets and potentially aerosols) and a friend (as a non-invasive diagnostic tool for viral detection and immune status surveillance) in combating COVID-19.
The coronavirus disease 2019 (COVID-19) outbreak, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became a global ongoing pandemic. Timely, accurate, and non-invasive SARS-CoV-2 detection in both symptomatic and asymptomatic patients, as well as the determination of their immune status, will facilitate effective large-scale pandemic control measures to prevent the spread of COVID-19. This review focuses on the role of saliva as both a foe (a common mode of viral transmission via salivary droplets and potentially aerosols) and a friend (as a non-invasive diagnostic tool for viral detection and immune status surveillance) in combating COVID-19.
This review explores the potential role of saliva in the COVID-19 pandemic, as both a mechanism for the spread of the disease and a readily accessible diagnostic tool for detecting the presence of the virus, as well as an individual’s immune status.
- Results: saliva can be used to diagnose the novel coronavirus infection, and even help monitor immunity to the virus.
- Saliva is a “potent” biofluid source option for the detection of SARS-CoV-2 since it is non-invasive, easy-to-access, and low-cost.
- Saliva can be stored at -80 °C for several years with little degradation.
- Researcher Quote: “Ironically, saliva is a leading way that disease is transmitted, via droplets on surfaces and in the air, but it can also be incredibly useful to us for diagnosing the virus and monitoring a person’s health,” said Dr. Pingping Han, a postdoctoral research fellow in UQ’s School of Dentistry. “Indeed, saliva may be useful for both diagnosing the presence and sequelae of COVID-19 infection, as well as identifying and tracking the development of immunity to the virus.”
Received: 21 April 2020 / Revised: 6 May 2020 / Accepted: 7 May 2020 / Published: 9 May 2020
Han, P.; Ivanovski, S. Saliva—Friend and Foe in the COVID-19 Outbreak. Diagnostics 2020, 10, 290.
Yale University
Saliva Offers Greater Detection Sensitivity & Consistency
 50 researchers at Yale University sought to validate saliva as a new diagnostic approach over nasopharyngeal swabs because swabs offer low sensitivity, exposure risks to healthcare workers, and global shortages of swabs and personal protective equipment.
50 researchers at Yale University sought to validate saliva as a new diagnostic approach over nasopharyngeal swabs because swabs offer low sensitivity, exposure risks to healthcare workers, and global shortages of swabs and personal protective equipment.
- Results: Saliva samples taken from just inside the mouth provided greater detection sensitivity and consistency throughout the course of an infection than the broadly recommended nasopharyngeal (NP) approach.
- The study also concluded: there was less variability in results with the self-sample collection of saliva.
- Researcher Quote: “Taken together, our findings demonstrate that saliva is a viable and more sensitive alternative to nasopharyngeal swabs and could enable at-home self-administered sample collection for accurate large-scale SARS-CoV-2 testing,” said first author Anne Wyllie, an associate research scientist at the Yale School of Public Health and a member of its Public Health Modeling Unit.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.


Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask the Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.





