SDNA Saliva Collection FDA 510(k) Class II Medical Device Clearance
PRESS RELEASE
Leslie Titus Bryant
February 22, 2023
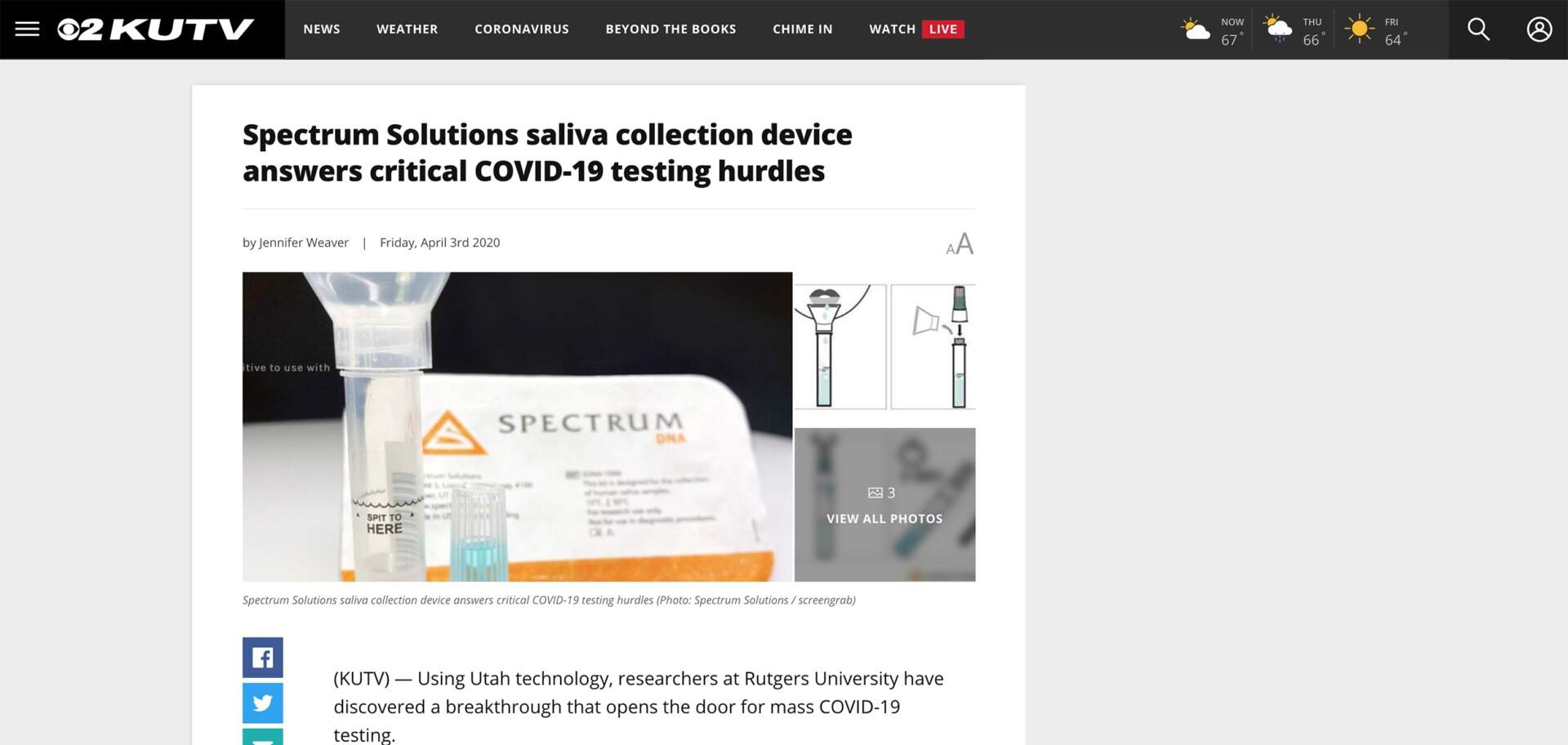
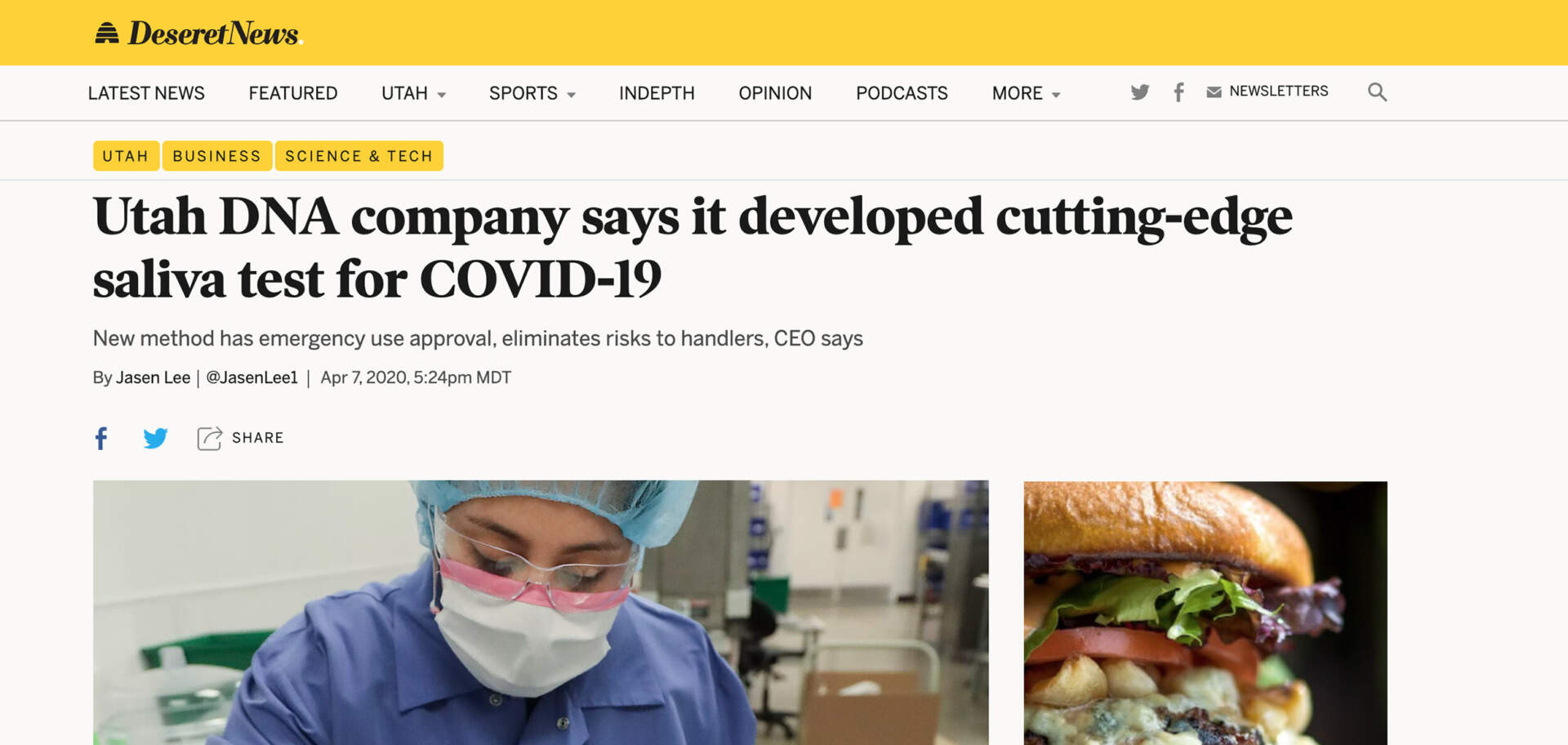
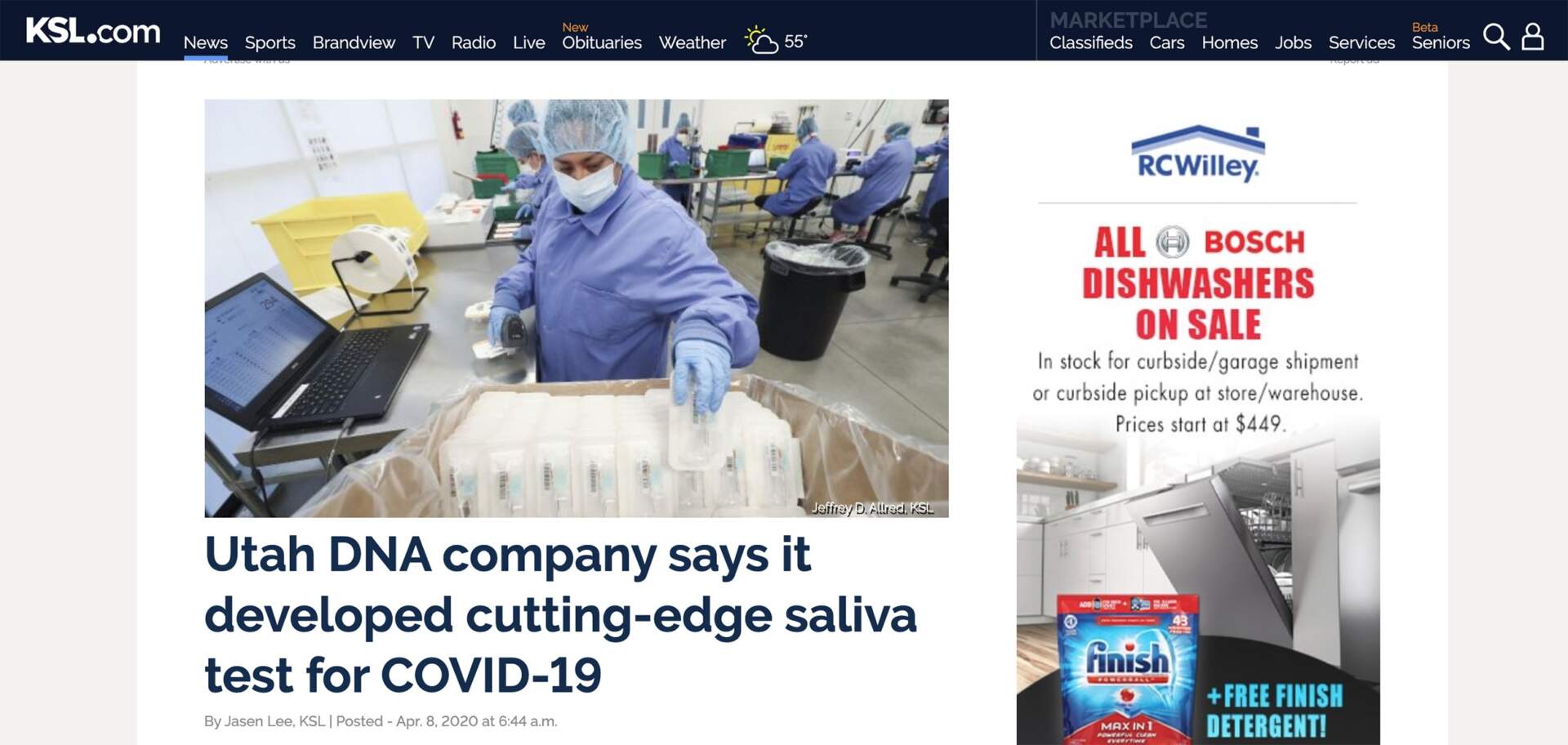
 SALT LAKE CITY, UTAH—Spectrum Solutions, LLC today announced the U.S. Food and Drug Administration (FDA) has granted 510(k) Class II clearance to the SDNA Saliva Collection Device as a microbial nucleic acid storage and stabilization device.
SALT LAKE CITY, UTAH—Spectrum Solutions, LLC today announced the U.S. Food and Drug Administration (FDA) has granted 510(k) Class II clearance to the SDNA Saliva Collection Device as a microbial nucleic acid storage and stabilization device.
The SDNA device is driving a new era of healthcare that turns to salivary collection and preservation as a means to detect viral infections and resolve the most common points of failure associated with whole saliva. The device is heralded for its ability to maximize detection at the lowest levels and neutralize viruses within 10 seconds of collection to mitigate unnecessary viral exposure. The company’s patented preservation media keeps analytes stable for many weeks at ambient temperatures, ensuring safe, easy, and secure specimen storage and transport. To ensure the highest degree of stringency and quality, automated methods for extraction and validation were performed on industry-leading platforms.
Near the beginning of the pandemic, Spectrum’s SDNA-1000 was the first to gain FDA Emergency Use Authorization (EUA) and introduce a nation, held under viral siege, to the noninvasive, highly accurate, and earliest detection benefits of COVID-19 testing using the self-collection of saliva. With over two years of processing SARS-CoV-2 saliva tests, the device and its patented nucleic acid preservation chemistry have not only demonstrated but proven their unique and superior capabilities deserving of this new device clearance.
“This 510(k) clearance and certification as an IVD molecular diagnostic device enables physicians, hospitals, researchers, and others to leverage our SDNA Saliva Collection Device in a broad array of FDA-cleared and LDT diagnostic testing applications,” said Stephen Fanning, CEO at Spectrum Solutions. “Those conducting screening and diagnostic tests can confidently and safely expand access and opportunity incorporating saliva as a primary or alternative biomaterial into their existing testing protocols.”
“We couldn’t be more excited for the opportunity this new 510(k) device clearance delivers in supporting laboratory medicine and healthcare systems in safely and confidently decentralizing specimen collections for precise and accurate testing,” said Chief Medical Officer, Rohit Gupta, at Spectrum Solutions.
Click here to view this press release on the Business Wire.
___
SDNA Saliva Collection FDA 510(k) Class II Medical Device Clearance
PRESS RELEASE
Leslie Titus Bryant
February 22, 2023
SALT LAKE CITY, UTAH—Spectrum Solutions, LLC today announced the U.S. Food and Drug Administration (FDA) has granted 510(k) Class II clearance to the SDNA Saliva Collection Device as a microbial nucleic acid storage and stabilization device.
 The SDNA device is driving a new era of healthcare that turns to salivary collection and preservation as a means to detect viral infections and resolve the most common points of failure associated with whole saliva. The device is heralded for its ability to maximize detection at the lowest levels and neutralize viruses within 10 seconds of collection to mitigate unnecessary viral exposure. The company’s patented preservation media keeps analytes stable for many weeks at ambient temperatures, ensuring safe, easy, and secure specimen storage and transport. To ensure the highest degree of stringency and quality, automated methods for extraction and validation were performed on industry-leading platforms.
The SDNA device is driving a new era of healthcare that turns to salivary collection and preservation as a means to detect viral infections and resolve the most common points of failure associated with whole saliva. The device is heralded for its ability to maximize detection at the lowest levels and neutralize viruses within 10 seconds of collection to mitigate unnecessary viral exposure. The company’s patented preservation media keeps analytes stable for many weeks at ambient temperatures, ensuring safe, easy, and secure specimen storage and transport. To ensure the highest degree of stringency and quality, automated methods for extraction and validation were performed on industry-leading platforms.
Near the beginning of the pandemic, Spectrum’s SDNA-1000 was the first to gain FDA Emergency Use Authorization (EUA) and introduce a nation, held under viral siege, to the noninvasive, highly accurate, and earliest detection benefits of COVID-19 testing using the self-collection of saliva. With over two years of processing SARS-CoV-2 saliva tests, the device and its patented nucleic acid preservation chemistry have not only demonstrated but proven their unique and superior capabilities deserving of this new device clearance.
“This 510(k) clearance and certification as an IVD molecular diagnostic device enables physicians, hospitals, researchers, and others to leverage our SDNA Saliva Collection Device in a broad array of FDA-cleared and LDT diagnostic testing applications,” said Stephen Fanning, CEO at Spectrum Solutions. “Those conducting screening and diagnostic tests can confidently and safely expand access and opportunity incorporating saliva as a primary or alternative biomaterial into their existing testing protocols.”
“We couldn’t be more excited for the opportunity this new 510(k) device clearance delivers in supporting laboratory medicine and healthcare systems in safely and confidently decentralizing specimen collections for precise and accurate testing,” said Chief Medical Officer, Rohit Gupta, at Spectrum Solutions.
Click here to view this press release on the Business Wire.
___

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask the Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com