FDA COVID-19 UPDATE:
THE NEW YORK TIMES: Just Spit and Wait – New Coronavirus Test Offers Advantage
For Immediate Release
May, 08, 2020
May, 08, 2020
Today, the U.S. Food and Drug Administration authorized the first diagnostic test with the option of using home-collected saliva samples for COVID-19 testing. Specifically, the FDA issued an emergency use authorization (EUA) to Rutgers Clinical Genomics Laboratory for their COVID-19 laboratory-developed test (LDT), which had been previously added to the high complexity molecular-based LDT “umbrella” EUA, to permit testing of samples self-collected by patients at home using the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device. This announcement builds on last month’s EUA for the first diagnostic test with a home-collection option, which uses a sample collected from the patient’s nose with a nasal swab and saline.
“Authorizing additional diagnostic tests with the option of at-home sample collection will continue to increase patient access to testing for COVID-19. This provides an additional option for the easy, safe and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site,” said FDA Commissioner Stephen M. Hahn, M.D. “We will continue to work around the clock to support the development of accurate and reliable tests, as we have done throughout this pandemic. The FDA has authorized more than 80 COVID-19 tests and adding more options for at-home sample collection is an important advancement in diagnostic testing during this public health emergency.”
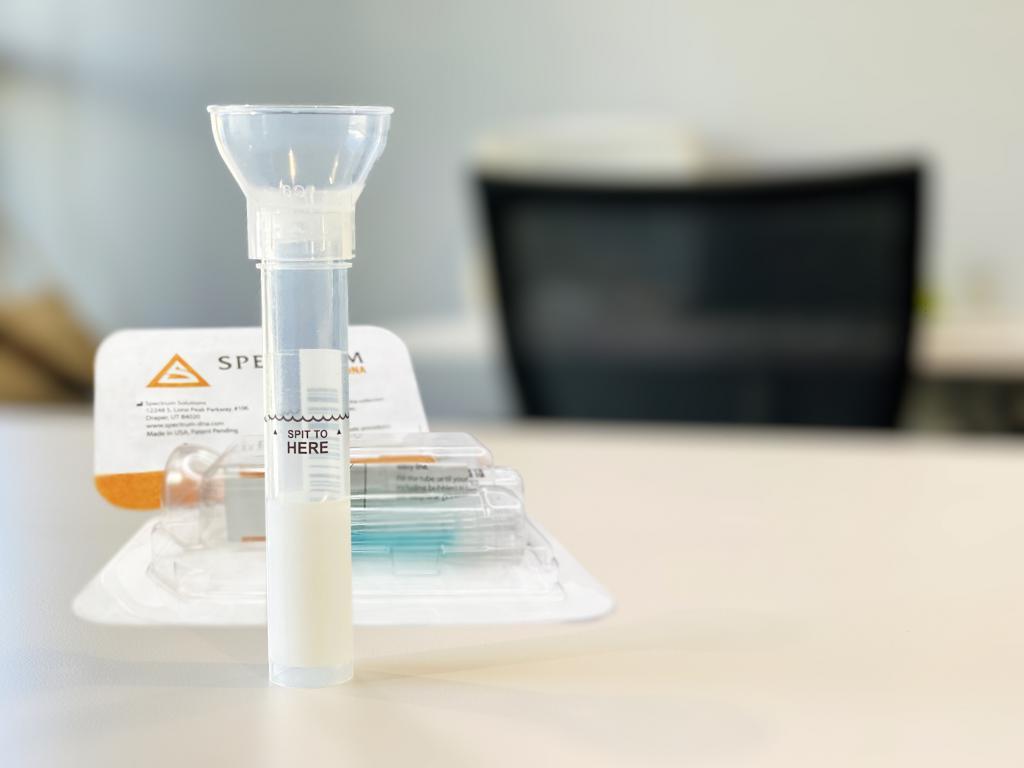
SDNA saliva collection devices at home. ©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Today, the U.S. Food and Drug Administration authorized the first diagnostic test with the option of using home-collected saliva samples for COVID-19 testing. Specifically, the FDA issued an emergency use authorization (EUA) to Rutgers Clinical Genomics Laboratory for their COVID-19 laboratory-developed test (LDT), which had been previously added to the high complexity molecular-based LDT “umbrella” EUA, to permit testing of samples self-collected by patients at home using the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device. This announcement builds on last month’s EUA for the first diagnostic test with a home-collection option, which uses a sample collected from the patient’s nose with a nasal swab and saline.
“Authorizing additional diagnostic tests with the option of at-home sample collection will continue to increase patient access to testing for COVID-19. This provides an additional option for the easy, safe and convenient collection of samples required for testing without traveling to a doctor’s office, hospital or testing site,” said FDA Commissioner Stephen M. Hahn, M.D. “We will continue to work around the clock to support the development of accurate and reliable tests, as we have done throughout this pandemic. The FDA has authorized more than 80 COVID-19 tests and adding more options for at-home sample collection is an important advancement in diagnostic testing during this public health emergency.”

SDNA saliva collection devices at home. ©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Today’s EUA permits testing saliva samples collected from the patient using a designated self-collection kit.
Today’s EUA for Rutgers Clinical Genomics Laboratory’s molecular test permits testing of a saliva sample collected from the patient using a designated self-collection kit. Once patients collect their saliva sample, they return it to the Rutgers Clinical Genomics Laboratory in a sealed package for testing.
The Rutgers Clinical Genomics Laboratory test is currently the only authorized COVID-19 diagnostic test that uses saliva samples to test for SARS-CoV-2, the strain of coronavirus that causes COVID-19. The test remains prescription only.
Today’s authorization is limited to testing performed at the Rutgers Clinical Genomics Laboratory using their molecular LDT COVID-19 authorized test for saliva specimens collected using the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device. It is important to note that this is not a general authorization for at-home collection of patient samples using other collection methods, saliva collection devices, or tests, or for tests fully conducted at home.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
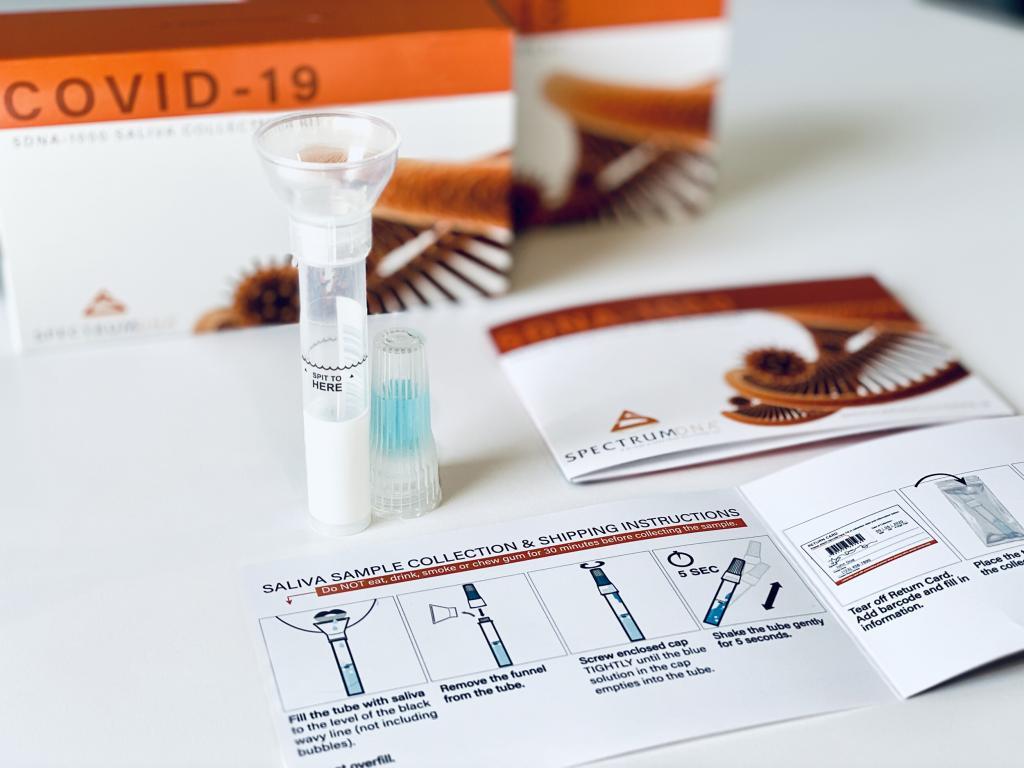
© Spectrum Solutions™ Saliva Collection Device | Photo Credit: Leslie Titus Bryant
Today’s EUA for Rutgers Clinical Genomics Laboratory’s molecular test permits testing of a saliva sample collected from the patient using a designated self-collection kit. Once patients collect their saliva sample, they return it to the Rutgers Clinical Genomics Laboratory in a sealed package for testing.
The Rutgers Clinical Genomics Laboratory test is currently the only authorized COVID-19 diagnostic test that uses saliva samples to test for SARS-CoV-2, the strain of coronavirus that causes COVID-19. The test remains prescription only.
Today’s authorization is limited to testing performed at the Rutgers Clinical Genomics Laboratory using their molecular LDT COVID-19 authorized test for saliva specimens collected using the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device. It is important to note that this is not a general authorization for at-home collection of patient samples using other collection methods, saliva collection devices, or tests, or for tests fully conducted at home.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.

© Spectrum Solutions™ Saliva Collection Device | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.