PRESS RELEASE
FDA Emergency Use Authorization Granted Utilizing Saliva for COVID-19 Testing Exclusively Using SDNA-1000 Saliva Collection Device From Spectrum Solutions
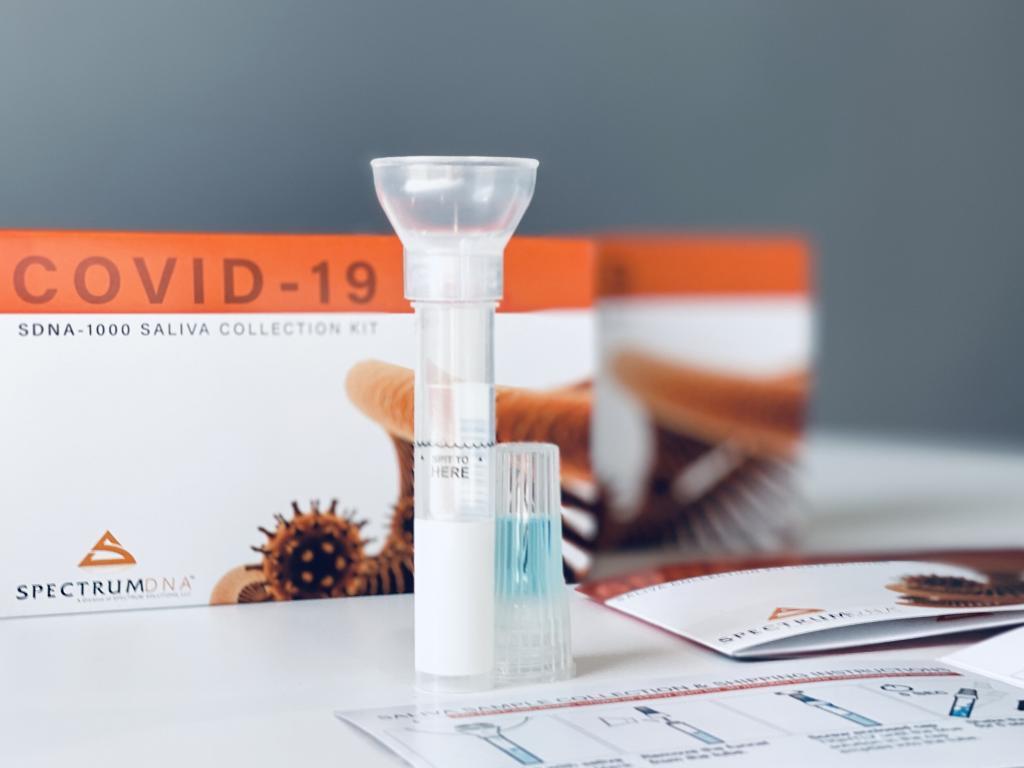
EUA200090 states, “Saliva specimens must be collected, transported and stored using the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device.”
We are excited to share the following press release and the unprecedented saliva testing discovery for COVID-19. The RUDCR study additionally provided further proof that the technically superior SDNA-1000 saliva collection device and its patented preservation solution, initially created for the preservation of DNA used in genetic and molecular diagnostic testing, can also be used to protect and preserve viral RNA used in the detection of infection.
“This announcement couldn’t have come at a better time,” Says Stephen Fanning, CEO of Spectrum Solutions. “The FDA EUA approval for the immediate use of saliva in COVID-19 testing means we can now help as many people as quickly as we can. The fact that it specifies the exclusive use of our SDNA-1000 means we will be working day and night to increase the capacity of our Utah manufacturing facility to more than triple its production of the SDNA-1000. We have already proven our ability to successfully scale production in order to meet extreme demands and we are up for this challenge as well.”
Press Release
Salt Lake City—April 13, 2020—Spectrum Solutions, LLC today announced the FDA Emergency Use Authorization (EUA) approval for the immediate use of saliva in COVID-19 testing exclusively using the Spectrum SDNA-1000 saliva collection device which has been proven to protect and preserve RNA used for the detection of infection.
The issued EUA for saliva as a viable biosample in COVID-19 testing came from the result of a detailed saliva vs. swab study strategically looking for an answer to the devastating swab and media supply shortages felt not only nationwide but globally. The study conclusion uncovered a novel, and technically robust testing alternative using saliva collected with Spectrum’s SDNA-1000 saliva collection device. These results fast-tracked EUA approval for RUCDR Infinite Biologics – Rutgers Clinical Genomics Laboratory and its Perkin Elmer nucleic acid extraction and ThermoFisher TaqPath SARS-CoV-2 automated processes using the Spectrum Solutions SDNA-1000 device.
“Using saliva to test for COVID-19 overcomes many of the challenges the nation faces that are inherent to current testing methods including supply shortages and risks to exposure for healthcare professionals,” said Stephen Fanning, CEO of Spectrum Solutions. “Now, under a medical professional’s direction, the saliva collection can be self-administered by individuals who may be in quarantine or self-isolation, removing the need to be in close contact with medical staff.”
“The Spectrum Solutions SDNA-1000 Saliva Collection Device was chosen to collect, transport and store saliva specimens for the TaqPath SARS-CoV-2 Assay proof-point study given RUCDR’s experience with Spectrum products across a wide range of applications currently employed,” said Dr. Andrew Brooks, Chief Operating Officer at RUCDR Infinite Biologics and Professor of Genetics at Rutgers University. “The preservation solution was a main focus of the study to ensure that the virus would be inactivated to the point that we would be able to maintain and stabilizes the RNA transcripts for sensitive and specific QPCR analysis in order to determine whether a person has the virus. The device’s intuitive ease-of-use to facilitate minimally supervised or in most cases complete self-collection is a tremendous advance to current COVID-19 sample collection strategies. Together these benefits will significantly add to expanding access to critical testing needs.”
About RUCDR
RUCDR Infinite Biologics, which is part of Rutgers’ Human Genetics Institute of New Jersey, is the world’s largest university-based cell and DNA repository. Its mission is to understand the genetic causes of common, complex diseases and to discover diagnoses, treatments, and cures for them. The organization collaborates with researchers in the public and private sectors throughout the world, providing the highest quality bio-banking services and biomaterials, as well as scientific and technical support. RUCDR clinical laboratory provides high throughput testing of high complexity diagnostic services to both government and industry partners. [Link to RUCDR Press Release]
About Spectrum Solutions and Spectrum DNA
Headquartered in Salt Lake City, Utah, Spectrum Solutions and its medical device and services division, Spectrum DNA, focus on innovative, end-to-end product development, manufacturing, and global fulfillment solutions. With concentrated industry expertise, Spectrum DNA specializes in engineering innovative molecular diagnostic solutions that simplify the biosample collection process while offering donors complete physical and digital chain-of-custody. With on-site production facilities, we are a single-source provider of full-service medical device manufacturing, custom, and private label packaging, kitting, and direct-to-donor global fulfillment. Our new biosample collection devices, patented technology, and services provide measurable process optimization, unprecedented efficiency, and unmatched global scalability.
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
EUA200090 states, “Saliva specimens must be collected, transported and stored using the Spectrum Solutions LLC SDNA-1000 Saliva Collection Device.”
We are excited to share the following press release and the unprecedented saliva testing discovery for COVID-19. The RUDCR study additionally provided further proof that the technically superior SDNA-1000 saliva collection device and its patented preservation solution, initially created for the preservation of DNA used in genetic and molecular diagnostic testing, can also be used to protect and preserve viral RNA used in the detection of infection.
“This announcement couldn’t have come at a better time,” Says Stephen Fanning, CEO of Spectrum Solutions. “The FDA EUA approval for the immediate use of saliva in COVID-19 testing means we can now help as many people as quickly as we can. The fact that it specifies the exclusive use of our SDNA-1000 means we will be working day and night to increase the capacity of our Utah manufacturing facility to more than triple its production of the SDNA-1000. We have already proven our ability to successfully scale production in order to meet extreme demands and we are up for this challenge as well.”
Press Release
Salt Lake City—April 13, 2020—Spectrum Solutions, LLC today announced the FDA Emergency Use Authorization (EUA) approval for the immediate use of saliva in COVID-19 testing exclusively using the Spectrum SDNA-1000 saliva collection device which has been proven to protect and preserve RNA used for the detection of infection.
The issued EUA for saliva as a viable biosample in COVID-19 testing came from the result of a detailed saliva vs. swab study strategically looking for an answer to the devastating swab and media supply shortages felt not only nationwide but globally. The study conclusion uncovered a novel, and technically robust testing alternative using saliva collected with Spectrum’s SDNA-1000 saliva collection device. These results fast-tracked EUA approval for RUCDR Infinite Biologics – Rutgers Clinical Genomics Laboratory and its Perkin Elmer nucleic acid extraction and ThermoFisher TaqPath SARS-CoV-2 automated processes using the Spectrum Solutions SDNA-1000 device.
“Using saliva to test for COVID-19 overcomes many of the challenges the nation faces that are inherent to current testing methods including supply shortages and risks to exposure for healthcare professionals,” said Stephen Fanning, CEO of Spectrum Solutions. “Now, under a medical professional’s direction, the saliva collection can be self-administered by individuals who may be in quarantine or self-isolation, removing the need to be in close contact with medical staff.”
“The Spectrum Solutions SDNA-1000 Saliva Collection Device was chosen to collect, transport and store saliva specimens for the TaqPath SARS-CoV-2 Assay proof-point study given RUCDR’s experience with Spectrum products across a wide range of applications currently employed,” said Dr. Andrew Brooks, Chief Operating Officer at RUCDR Infinite Biologics and Professor of Genetics at Rutgers University. “The preservation solution was a main focus of the study to ensure that the virus would be inactivated to the point that we would be able to maintain and stabilizes the RNA transcripts for sensitive and specific QPCR analysis in order to determine whether a person has the virus. The device’s intuitive ease-of-use to facilitate minimally supervised or in most cases complete self-collection is a tremendous advance to current COVID-19 sample collection strategies. Together these benefits will significantly add to expanding access to critical testing needs.”
About RUCDR
RUCDR Infinite Biologics, which is part of Rutgers’ Human Genetics Institute of New Jersey, is the world’s largest university-based cell and DNA repository. Its mission is to understand the genetic causes of common, complex diseases and to discover diagnoses, treatments, and cures for them. The organization collaborates with researchers in the public and private sectors throughout the world, providing the highest quality bio-banking services and biomaterials, as well as scientific and technical support. RUCDR clinical laboratory provides high throughput testing of high complexity diagnostic services to both government and industry partners. [Link to RUCDR Press Release]
About Spectrum Solutions and Spectrum DNA
Headquartered in Salt Lake City, Utah, Spectrum Solutions and its medical device and services division, Spectrum DNA, focus on innovative, end-to-end product development, manufacturing, and global fulfillment solutions. With concentrated industry expertise, Spectrum DNA specializes in engineering innovative molecular diagnostic solutions that simplify the biosample collection process while offering donors complete physical and digital chain-of-custody. With on-site production facilities, we are a single-source provider of full-service medical device manufacturing, custom, and private label packaging, kitting, and direct-to-donor global fulfillment. Our new biosample collection devices, patented technology, and services provide measurable process optimization, unprecedented efficiency, and unmatched global scalability.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.

