FORBES:
WASHINGTON POST
HEALTH 202: The newest way to test for coronavirus: Spitting in a tube
HEALTH 202: The newest way to test for coronavirus: Spitting in a tube
By Lisette Voytko, Forbes Staff
Breaking News: April 15, 2020
Breaking News: April 15, 2020
TOPLINE: The first U.S. coronavirus saliva testing site opens Wednesday in New Jersey after the Food and Drug Administration granted emergency authorization for the method, which healthcare experts say is quicker, more scalable and safer to administer as the virus continues to spread across the country.
KEY FACTS
- The saliva testing method was developed by a Rutgers University lab, NBC New York reported, and the FDA granted emergency authorization for it on Friday.
- President Trump hailed the saliva test during his Tuesday press briefing: “I call it innovation under pressure.”
- Patients take the test by spitting into a saliva collection device, which saves swabs used for nasal tests and decreases the amount of contact between healthcare workers and patients.
- A proprietary solution detects the virus in the saliva, according to NBC, and the turnaround for results is 24 to 48 hours.
- Thousands of saliva samples can be processed daily, said Stephen Fanning, president of Utah Spectrum Solutions, a biotech firm that worked on the saliva test.
- It remains unclear if enough of the virus will be present in saliva to produce consistently reliable results, ABC News reported, but a Rutgers review of a small sample size submitted to the FDA showed the saliva test was 100% as reliable as nasal swab testing.
CRUCIAL QUOTE
“We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as self-collection of saliva is more quick and scalable than swab collections,” said Andrew Brooks, a senior official at the Rutgers lab, in a press release. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
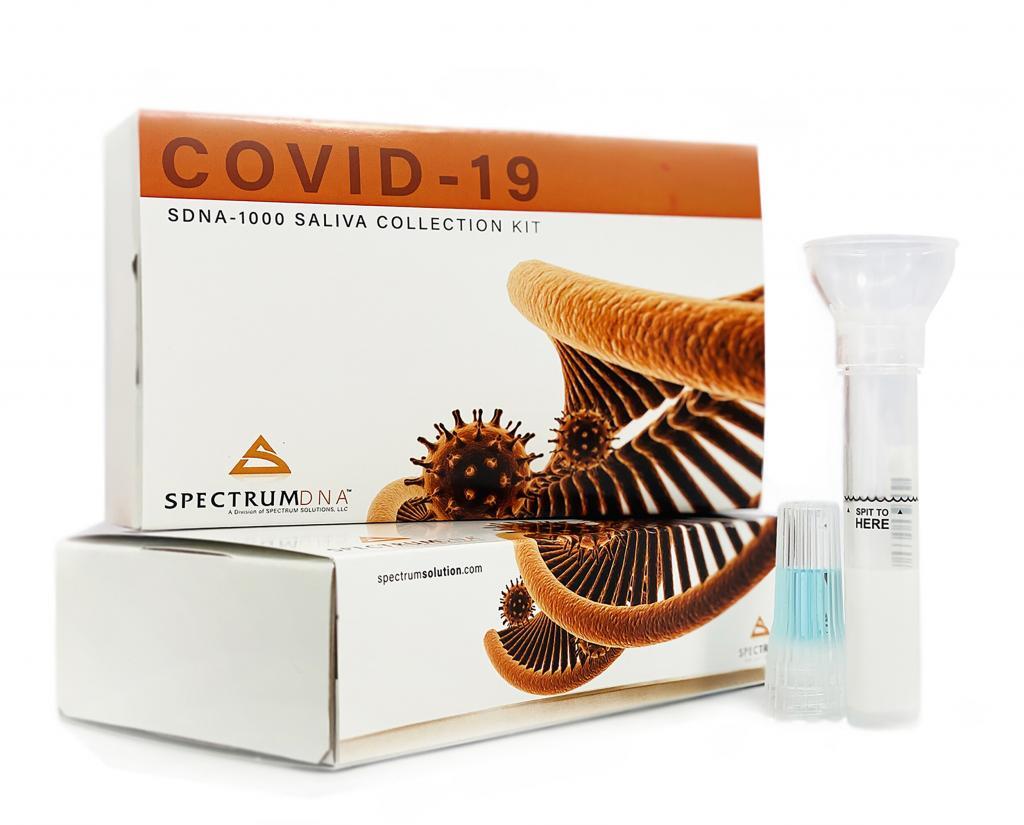
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
KEY FACTS
- The saliva testing method was developed by a Rutgers University lab, NBC New York reported, and the FDA granted emergency authorization for it on Friday.
- President Trump hailed the saliva test during his Tuesday press briefing: “I call it innovation under pressure.”
- Patients take the test by spitting into a saliva collection device, which saves swabs used for nasal tests and decreases the amount of contact between healthcare workers and patients.
- A proprietary solution detects the virus in the saliva, according to NBC, and the turnaround for results is 24 to 48 hours.
- Thousands of saliva samples can be processed daily, said Stephen Fanning, president of Utah Spectrum Solutions, a biotech firm that worked on the saliva test.
- It remains unclear if enough of the virus will be present in saliva to produce consistently reliable results, ABC News reported, but a Rutgers review of a small sample size submitted to the FDA showed the saliva test was 100% as reliable as nasal swab testing.
CRUCIAL QUOTE
“We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as self-collection of saliva is more quick and scalable than swab collections,” said Andrew Brooks, a senior official at the Rutgers lab, in a press release. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.


