GENETIC ENGINEERING NEWS:
SARS-CoV-2 Detection in saliva samples are more sensitive and consistent
Published: April 27, 2020

The test, developed by a Rutgers lab, could be a safer and faster alternative.
Researchers are working around the clock, innovating ways to improve the COVID-19 testing process. Recently, a group of researchers from Yale University presented findings that suggest that one improvement could be made right at the beginning of the testing process—in the choice of the sample to be tested. Although the current gold standard is to use samples collected from nasopharyngeal swabs (NPS), the group found that using saliva for SARS-CoV-2 detection is more sensitive and consistent than using a nasopharyngeal swab. They assert that saliva should be considered as a reliable sample type to improve COVID-19 testing.
The work is published as a preprint in MedRxiv. The title of the preprint (a paper that has not yet gone through the peer review process) is “Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs.”
Currently available SARS-CoV-2 detection tests suffer from multiple challenges including low sensitivity and reliance on swabs. In addition, swab collection requires close contact between the healthcare worker performing the collection and the patient, which poses a health risk to healthcare workers. Using saliva for COVID-19 testing has several advantages when compared to collection of nasopharyngeal swabs. Saliva collection does not require specialized swabs or tubes, is far less uncomfortable for the patient, and may even be done independently by the patient–potentially alleviating the current overwhelming demand for healthcare workers. Indeed, in this study, the saliva samples were self-collected by the patient. The process is described as, “upon waking, patients were asked to avoid food, water, and brushing of teeth until the sample was collected. Patients were asked to repeatedly spit into a sterile urine cup until roughly a third full of liquid (excluding bubbles), before securely closing it.”
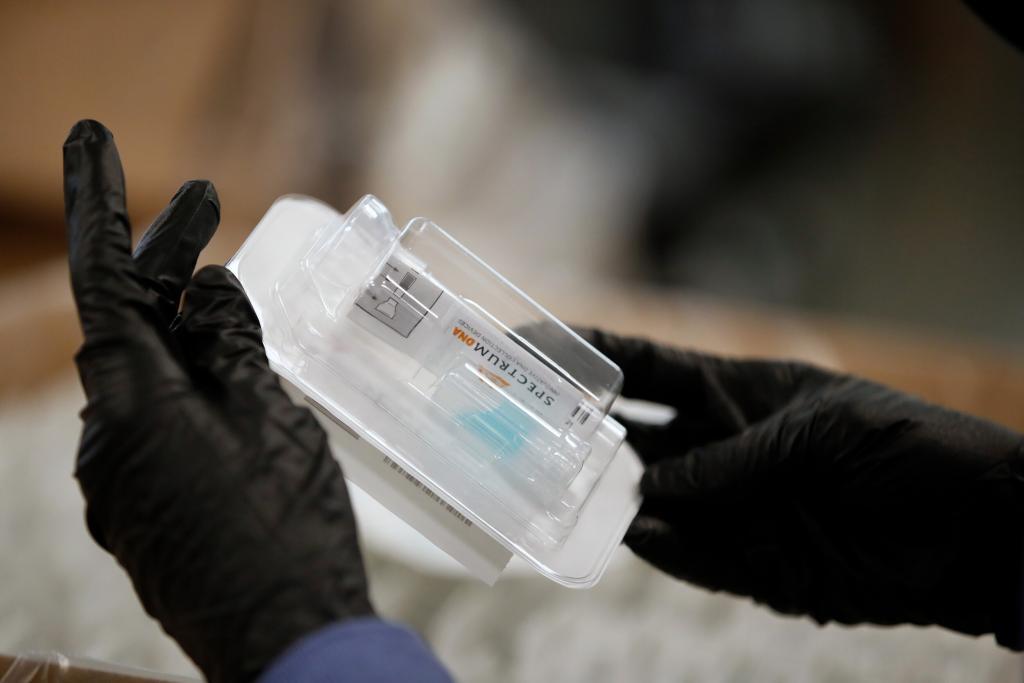
© Spectrum Solutions™ Bloomberg News/George Frey
The test, developed by a Rutgers lab, could be a safer and faster alternative.
Researchers are working around the clock, innovating ways to improve the COVID-19 testing process. Recently, a group of researchers from Yale University presented findings that suggest that one improvement could be made right at the beginning of the testing process—in the choice of the sample to be tested. Although the current gold standard is to use samples collected from nasopharyngeal swabs (NPS), the group found that using saliva for SARS-CoV-2 detection is more sensitive and consistent than using a nasopharyngeal swab. They assert that saliva should be considered as a reliable sample type to improve COVID-19 testing.
The work is published as a preprint in MedRxiv. The title of the preprint (a paper that has not yet gone through the peer review process) is “Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs.”
Currently available SARS-CoV-2 detection tests suffer from multiple challenges including low sensitivity and reliance on swabs. In addition, swab collection requires close contact between the healthcare worker performing the collection and the patient, which poses a health risk to healthcare workers. Using saliva for COVID-19 testing has several advantages when compared to collection of nasopharyngeal swabs. Saliva collection does not require specialized swabs or tubes, is far less uncomfortable for the patient, and may even be done independently by the patient–potentially alleviating the current overwhelming demand for healthcare workers. Indeed, in this study, the saliva samples were self-collected by the patient. The process is described as, “upon waking, patients were asked to avoid food, water, and brushing of teeth until the sample was collected. Patients were asked to repeatedly spit into a sterile urine cup until roughly a third full of liquid (excluding bubbles), before securely closing it.”

© Spectrum Solutions™ Bloomberg News/George Frey
Saliva is a better diagnostic sample for COVID-19 testing
The researchers evaluated SARS-CoV-2 detection in paired nasopharyngeal swabs and saliva samples collected from COVID-19 inpatients and asymptomatic healthcare workers at moderate-to-high risk of COVID-19 exposure. They collected clinical samples from 44 COVID-19 inpatient study participants—all of whom had serious illness—and 98 health care workers. After testing for the presence of the SARS-CoV-2 virus, the findings suggest that saliva samples provided greater detection, sensitivity, and consistency throughout the course of infection than the nasopharyngeal swabs.
This is a “super useful new study,” tweeted Akiko Iwasaki, PhD, professor at the Yale School of Medicine, showing that “saliva is a better diagnostic sample for COVID-19 testing than nasopharyngeal swabs.”
When the authors compared SARS-CoV-2 detection from patient-matched nasopharyngeal and saliva samples, they found that “saliva yielded greater detection sensitivity and consistency throughout the course of infection.” In addition, there was less variability in self-sample collection of saliva.
The experiment also detected SARS-CoV-2 from the saliva of two asymptomatic healthcare workers who tested negative from their nasopharyngeal swabs. This finding, tweeted Anne Wyllie, PhD, associate research scientist in epidemiology at Yale School of Medicine and lead author on the study, “suggests that saliva could be a viable alternative for identifying mild or subclinical infections.”
With further validation, Wyllie asserts, “widespread use of saliva sampling could be transformative for public health efforts.”
A group of researchers from Melbourne, Australia, published similar findings last week in a paper, “Saliva as a non-invasive specimen for detection of SARS-CoV-2,” published in the Journal of Clinical Microbiology as a Letter to the Editor.
This study used samples taken between March 25 and April 1. During that time, 622 patients were tested for COVID-19 at the Royal Melbourne Hospital through the screening clinic. All patients had nasopharyngeal swab samples, and 522/622 (83.9%) patients also provided saliva.

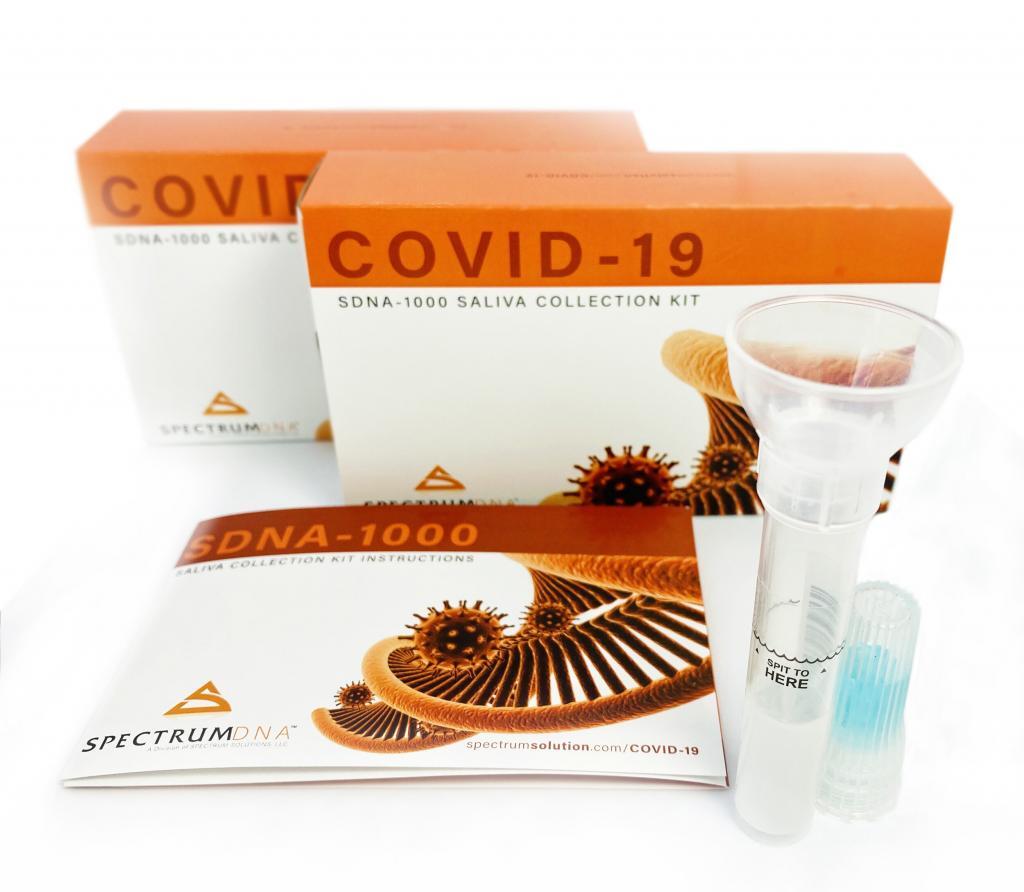
© Spectrum Solutions™ COVID-19 SDNA-1000 Saliva Collection Testing Kit | Bloomberg News/George Frey
The researchers evaluated SARS-CoV-2 detection in paired nasopharyngeal swabs and saliva samples collected from COVID-19 inpatients and asymptomatic healthcare workers at moderate-to-high risk of COVID-19 exposure. They collected clinical samples from 44 COVID-19 inpatient study participants—all of whom had serious illness—and 98 health care workers. After testing for the presence of the SARS-CoV-2 virus, the findings suggest that saliva samples provided greater detection, sensitivity, and consistency throughout the course of infection than the nasopharyngeal swabs.
This is a “super useful new study,” tweeted Akiko Iwasaki, PhD, professor at the Yale School of Medicine, showing that “saliva is a better diagnostic sample for COVID-19 testing than nasopharyngeal swabs.”
When the authors compared SARS-CoV-2 detection from patient-matched nasopharyngeal and saliva samples, they found that “saliva yielded greater detection sensitivity and consistency throughout the course of infection.” In addition, there was less variability in self-sample collection of saliva.
The experiment also detected SARS-CoV-2 from the saliva of two asymptomatic healthcare workers who tested negative from their nasopharyngeal swabs. This finding, tweeted Anne Wyllie, PhD, associate research scientist in epidemiology at Yale School of Medicine and lead author on the study, “suggests that saliva could be a viable alternative for identifying mild or subclinical infections.”
With further validation, Wyllie asserts, “widespread use of saliva sampling could be transformative for public health efforts.”
A group of researchers from Melbourne, Australia, published similar findings last week in a paper, “Saliva as a non-invasive specimen for detection of SARS-CoV-2,” published in the Journal of Clinical Microbiology as a Letter to the Editor.
This study used samples taken between March 25 and April 1. During that time, 622 patients were tested for COVID-19 at the Royal Melbourne Hospital through the screening clinic. All patients had nasopharyngeal swab samples, and 522/622 (83.9%) patients also provided saliva.

© Spectrum Solutions™ COVID-19 SDNA-1000 Saliva Collection Testing Kit | Bloomberg News/George Frey
Saliva could be a viable alternative for identifying mild or subclinical infections
Of the 622 people, 39 (6.3%) patients tested positive using standard RT-PCR tests on nasopharyngeal samples. Out of the 39 COVID-19 positive patients, SARS-CoV-2 was also detected in the saliva of 33 of them. As a control, the group also tested saliva specimens from 50 patients with PCR-negative swabs. SARS-CoV-2 was detected in 1 of the 50 samples.
Although the sensitivity of saliva as a diagnostic specimen was less than nasopharyngeal swabs in this study, the authors write that “saliva testing may be a suitable alternative first-line screening test in several environments, including low resource settings, with NPS reserved for patients with an ongoing high clinical index of suspicion. These findings are highly relevant in the face of shortages of both swabs and personal protective equipment in many settings.”
Indeed, saliva-based tests are moving into the COVID-19 testing arena as a saliva SARS-CoV-2 detection assay has already gained authorization through the U.S. Food and Drug Administration emergency use authorization. The test is from the Rutgers Clinical Genomics Laboratory.
The recent findings from both the Yale and Melbourne researchers amplify the call for immediate validation and implementation of saliva sampling for SARS-CoV-2.
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Of the 622 people, 39 (6.3%) patients tested positive using standard RT-PCR tests on nasopharyngeal samples. Out of the 39 COVID-19 positive patients, SARS-CoV-2 was also detected in the saliva of 33 of them. As a control, the group also tested saliva specimens from 50 patients with PCR-negative swabs. SARS-CoV-2 was detected in 1 of the 50 samples.
Although the sensitivity of saliva as a diagnostic specimen was less than nasopharyngeal swabs in this study, the authors write that “saliva testing may be a suitable alternative first-line screening test in several environments, including low resource settings, with NPS reserved for patients with an ongoing high clinical index of suspicion. These findings are highly relevant in the face of shortages of both swabs and personal protective equipment in many settings.”
Indeed, saliva-based tests are moving into the COVID-19 testing arena as a saliva SARS-CoV-2 detection assay has already gained authorization through the U.S. Food and Drug Administration emergency use authorization. The test is from the Rutgers Clinical Genomics Laboratory.
The recent findings from both the Yale and Melbourne researchers amplify the call for immediate validation and implementation of saliva sampling for SARS-CoV-2.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.

