KSL TOP STORY:
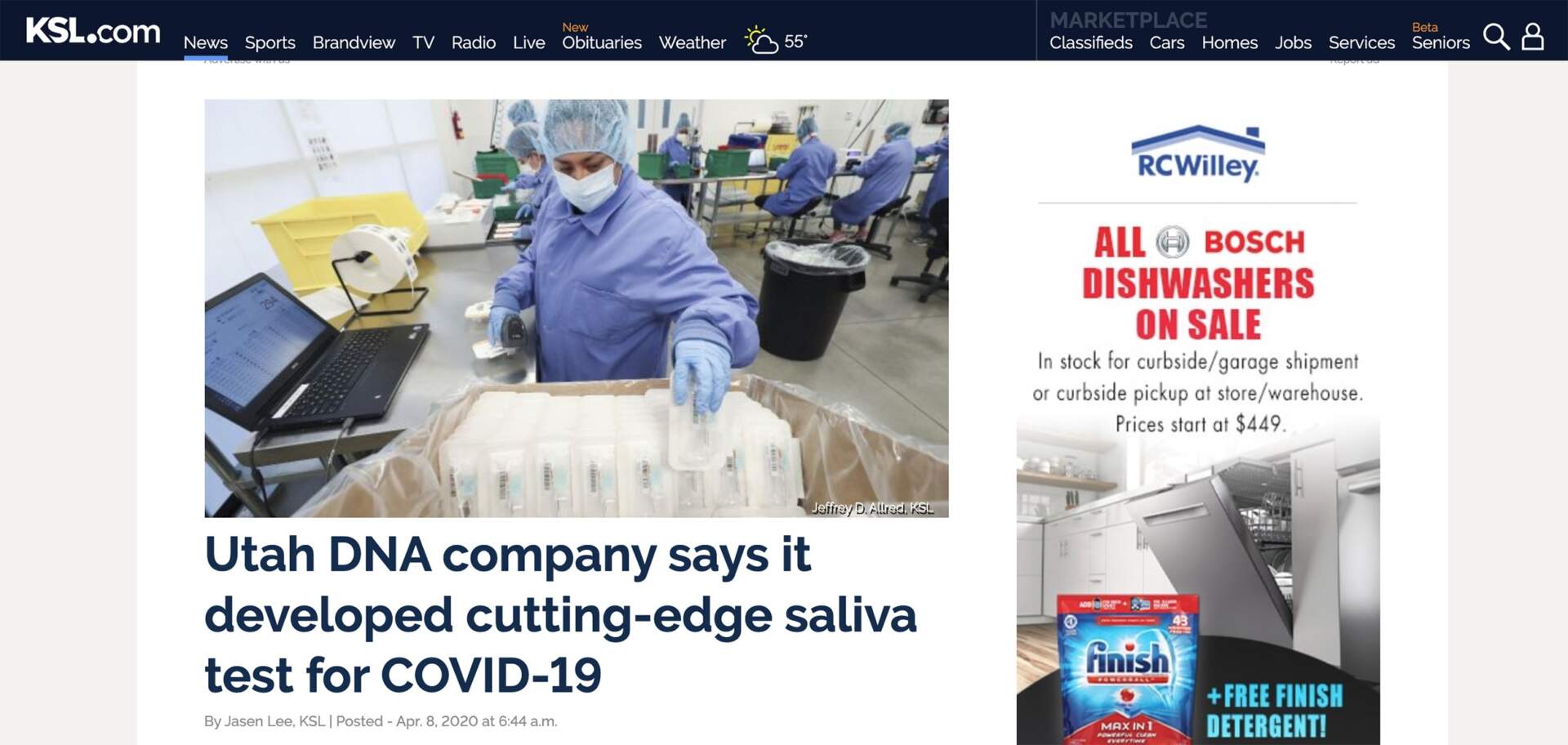
Utah DNA company says it developed cutting-edge saliva test for COVID-19
By Jasen Lee, KSL
Published: April 8, 2020
Published: April 8, 2020
(KSL NEWS – DRAPER, UT) — A Utah company says it has developed a test that may allow virtually anyone to find out whether they have the new coronavirus.
Scientists at Spectrum DNA have repurposed technology initially created to help users trace their ancestral lineage through saliva to now be used to detect COVID-19. Until recently, testing for the coronavirus required individuals to undergo invasive and often painful nasal and throat swabbing to gathering biologic material necessary for examination.
“We ran a study side by side, taking the swabs — either nasal swabs or throat swabs — and we looked at those versus looking at the saliva test, and it was 100% accurate in predicting if you had the virus or not,” said Stephen Fanning, CEO of Spectrum Solutions, which is headquartered in Draper.
The company said saliva testing is a new development that, when administered under the direction of a qualified medical professional, will allow for self-administered sample collection by individuals who may be in quarantine or self-isolation or for those showing no outward signs of illness.
“We ran a study side by side, taking the swabs — either nasal swabs or throat swabs — and we looked at those versus looking at the saliva test, and it was 100% accurate in predicting if you had the virus or not,” said Stephen Fanning, CEO of Spectrum Solutions, which is headquartered in Draper.
“This is a significant improvement in a couple of ways. Obviously, putting a very long swab in your nose is not easy (and) it’s rather painful, so with ours, there’s less pain,” he said. “No. 2, to do the swab method, you need to have a professional person stand there to insert the swab into the nasal cavity. Obviously, that potentially puts the professional at risk of getting the virus.”
The new method, which involves using a proprietary preservative solution, eliminates the risk to those handling the samples, Fanning said.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
(KSL NEWS – DRAPER, UT) — A Utah company says it has developed a test that may allow virtually anyone to find out whether they have the new coronavirus.
Scientists at Spectrum DNA have repurposed technology initially created to help users trace their ancestral lineage through saliva to now be used to detect COVID-19. Until recently, testing for the coronavirus required individuals to undergo invasive and often painful nasal and throat swabbing to gathering biologic material necessary for examination.
“We ran a study side by side, taking the swabs — either nasal swabs or throat swabs — and we looked at those versus looking at the saliva test, and it was 100% accurate in predicting if you had the virus or not,” said Stephen Fanning, CEO of Spectrum Solutions, which is headquartered in Draper.
The company said saliva testing is a new development that, when administered under the direction of a qualified medical professional, will allow for self-administered sample collection by individuals who may be in quarantine or self-isolation or for those showing no outward signs of illness.
“We ran a study side by side, taking the swabs — either nasal swabs or throat swabs — and we looked at those versus looking at the saliva test, and it was 100% accurate in predicting if you had the virus or not,” said Stephen Fanning, CEO of Spectrum Solutions, which is headquartered in Draper.
“This is a significant improvement in a couple of ways. Obviously, putting a very long swab in your nose is not easy (and) it’s rather painful, so with ours, there’s less pain,” he said. “No. 2, to do the swab method, you need to have a professional person stand there to insert the swab into the nasal cavity. Obviously, that potentially puts the professional at risk of getting the virus.”
The new method, which involves using a proprietary preservative solution, eliminates the risk to those handling the samples, Fanning said.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.