PRESS RELEASE:
Spectrum Solutions Receives CE Mark for its SDNA Saliva Collection Devices used for IVD Molecular Diagnostic Testing
Leslie Titus Bryant, Director of Marketing & Brand
January 06, 2021
| Leslie Titus Bryant, Director of Marketing & Brand January 06, 2021 |
|---|
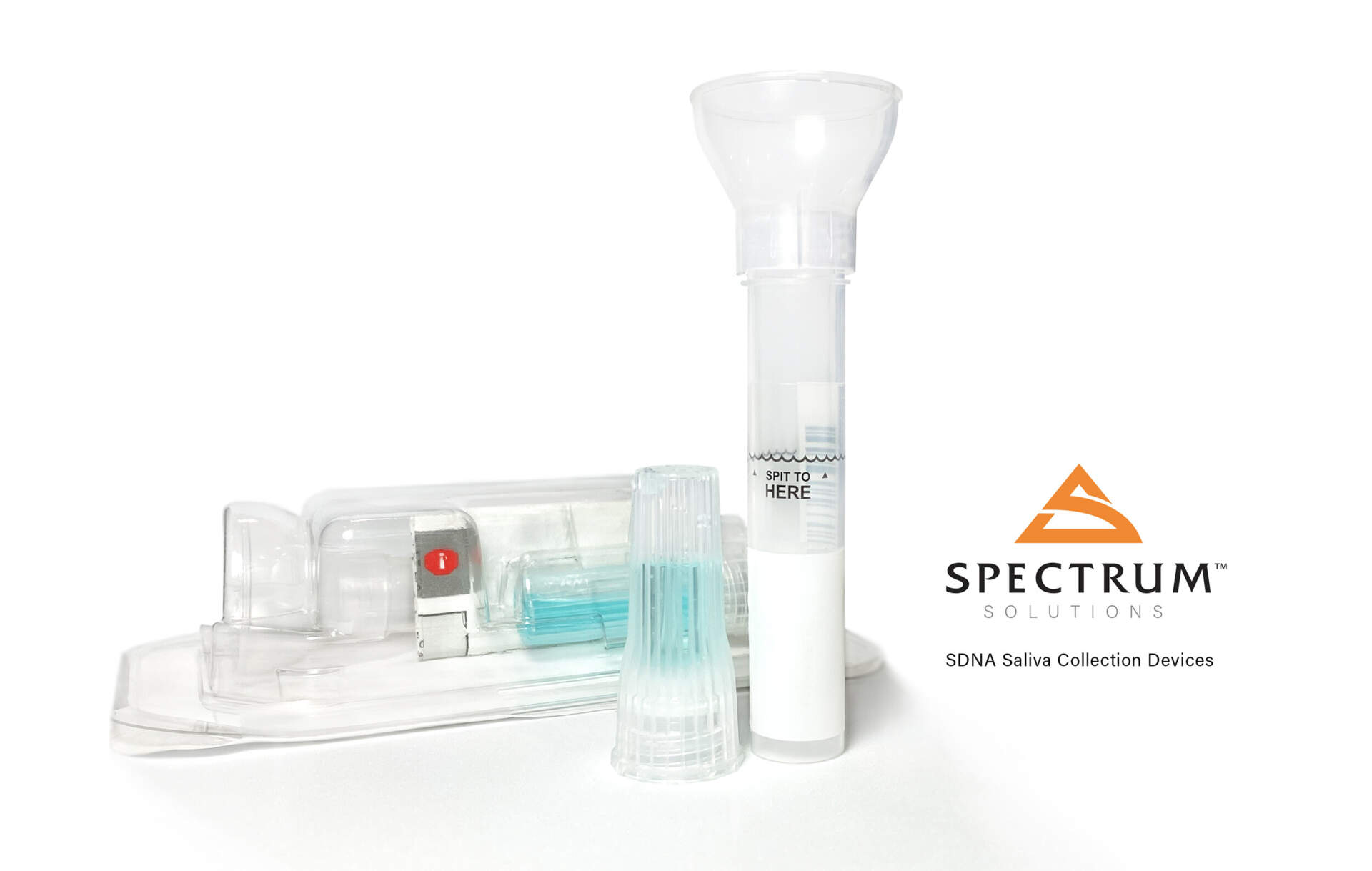
Spectrum Solutions, LLC today announced receiving CE Mark approval for the sale and distribution of its groundbreaking SDNA saliva collection device for IVD molecular diagnostic testing throughout Europe. The European CE Mark represents a standard of excellence and regulatory validation required to sell medical devices within the European Economic area. The equivalent mark within the United States would be an FDA medical device 510k approval. This mark demonstrates a conformity with health, safety, and environmental protection standards for products sold within Europe. The Spectrum SDNA-2000 model has been approved for integration in over the counter at-home testing kits with the SDNA-3000 model approved for prescription-only molecular diagnostic IVD testing applications.
“Receiving CE Mark approval for the SDNA-2000 and SDNA-3000 is a major milestone for our company and this offers physicians, healthcare organizations, governments, and individuals needing repeat IVD diagnostic testing an easy and pain-free option,” said Stephen Fanning, CEO of Spectrum Solutions. “At-home bio-sample self-collection means staying home when you don’t feel good and limiting exposure to other harmful illnesses for those most at risk.”
The Spectrum Solutions SDNA Saliva Collection Device was the first saliva-based diagnostic testing solution to receive the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for COVID-19 testing in the United States.
Bio-samples collected with the SDNA saliva collection system consistently demonstrate higher levels of testing accuracy, provide the safest & most robust testing biomaterial, is able to pinpoint an infection’s lifecycle stage, and delivers over 10 days of post-collection stability with no degradation in sample efficacy. The device’s intuitive ease-of-use design was engineered to facilitate unsupervised testing sample self-collection virtually eliminating user collection errors.
PRESS RELEASE:
Spectrum Solutions Receives CE Mark for its SDNA Saliva Collection Devices used for IVD Molecular Diagnostic Testing
Leslie Titus Bryant, Director of Marketing & Brand
January 06, 2021
| Leslie Titus Bryant, Director of Marketing & Brand January 06, 2021 |
|---|
Spectrum Solutions, LLC today announced receiving CE Mark approval for the sale and distribution of its groundbreaking SDNA saliva collection device for IVD molecular diagnostic testing throughout Europe. The European CE Mark represents a standard of excellence and regulatory validation required to sell medical devices within the European Economic area. The equivalent mark within the United States would be an FDA medical device 510k approval. This mark demonstrates a conformity with health, safety, and environmental protection standards for products sold within Europe. The Spectrum SDNA-2000 model has been approved for integration in over the counter at-home testing kits with the SDNA-3000 model approved for prescription-only molecular diagnostic IVD testing applications.
“Receiving CE Mark approval for the SDNA-2000 and SDNA-3000 is a major milestone for our company and this offers physicians, healthcare organizations, governments, and individuals needing repeat IVD diagnostic testing an easy and pain-free option,” said Stephen Fanning, CEO of Spectrum Solutions. “At-home bio-sample self-collection means staying home when you don’t feel good and limiting exposure to other harmful illnesses for those most at risk.”
The Spectrum Solutions SDNA Saliva Collection Device was the first saliva-based diagnostic testing solution to receive the U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for COVID-19 testing in the United States.
Bio-samples collected with the SDNA saliva collection system consistently demonstrate higher levels of testing accuracy, provide the safest & most robust testing biomaterial, is able to pinpoint an infection’s lifecycle stage, and delivers over 10 days of post-collection stability with no degradation in sample efficacy. The device’s intuitive ease-of-use design was engineered to facilitate unsupervised testing sample self-collection virtually eliminating user collection errors.


Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask the Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.


