THE WASHINGTON POST:
THE NEW YORK TIMES: Just Spit and Wait – New Coronavirus Test Offers Advantage
By Steven Mufson
Published: May 13, 2020
Published: May 13, 2020
Andrew Brooks, a Rutgers University molecular neuroscientist, remembers clearly having a long nasopharyngeal swab stuck up his nose in search of evidence of a virus. “It was terrible,” he recalls. “It felt like someone was poking the front of my brain.”
Now Brooks, who is also the chief operating officer and director of technology development at a firm called RUCDR Infinite Biologics, has come up with a coronavirus test that relies on nothing more than spitting into a cup.
His firm has won emergency use authorization from the Food and Drug Administration for saliva tests that people can perform at home. It’s a breakthrough in a nation that has struggled to expand coronavirus testing to the degree experts say is needed to safely return the country to some degree of normalcy.
Unlike tests conducted with nasal swabs, the saliva test does not require travel to a testing center. There are no perilous face-to-face encounters with technicians wearing masks that have been hard to come by. And there’s no need for the swabs that have also been in short supply. It’s just spit and mail to the Rutgers clinical genomics laboratory, with results within 48 hours.
“Rutgers blazed the path for home collection . . . Saliva is a big leap forward relative to swabs, and it is likely to play a major role in getting America back to work,” said Bob Terbrueggen, chief executive of DxTerity, a firm focused on tests for workplaces.
Even as state and local governments continue to buy traditional coronavirus testing kits that rely on nasopharyngeal swabs, major research universities and their private-sector partners are trying to leapfrog ahead to the next generation of tests.

Dr. Andrew Brooks, Chief Operating Officer at RUCDR Infinite Biologics is processing Spectrum saliva collection devices which enable RUCDR to screen a much broader population without putting health care professionals at risk. Self-collection of saliva is vital in reducing the spread of COVID-19 as well as preserving personal protective equipment necessary for safe patient care.
Andrew Brooks, a Rutgers University molecular neuroscientist, remembers clearly having a long nasopharyngeal swab stuck up his nose in search of evidence of a virus. “It was terrible,” he recalls. “It felt like someone was poking the front of my brain.”
Now Brooks, who is also the chief operating officer and director of technology development at a firm called RUCDR Infinite Biologics, has come up with a coronavirus test that relies on nothing more than spitting into a cup.
His firm has won emergency use authorization from the Food and Drug Administration for saliva tests that people can perform at home. It’s a breakthrough in a nation that has struggled to expand coronavirus testing to the degree experts say is needed to safely return the country to some degree of normalcy.
Unlike tests conducted with nasal swabs, the saliva test does not require travel to a testing center. There are no perilous face-to-face encounters with technicians wearing masks that have been hard to come by. And there’s no need for the swabs that have also been in short supply. It’s just spit and mail to the Rutgers clinical genomics laboratory, with results within 48 hours.
“Rutgers blazed the path for home collection . . . Saliva is a big leap forward relative to swabs, and it is likely to play a major role in getting America back to work,” said Bob Terbrueggen, chief executive of DxTerity, a firm focused on tests for workplaces.
Even as state and local governments continue to buy traditional coronavirus testing kits that rely on nasopharyngeal swabs, major research universities and their private-sector partners are trying to leapfrog ahead to the next generation of tests.

Dr. Andrew Brooks, Chief Operating Officer at RUCDR Infinite Biologics is processing Spectrum saliva collection devices which enable RUCDR to screen a much broader population without putting health care professionals at risk. Self-collection of saliva is vital in reducing the spread of COVID-19 as well as preserving personal protective equipment necessary for safe patient care.
Saliva creates the scenario that brings the test to the patient
Researchers from the University of Colorado at Boulder have launched a firm called Darwin Biosciences and are developing the “SickStick,” a device to measure the presence of the virus in saliva. Oklahoma State University, while awaiting FDA authorization, is using saliva to test thousands of nursing home patients. And in Connecticut, scientists are working on a test strip that could be taken at home for immediate results, without having to ship it to a lab — akin to a home pregnancy test.
[The newest way to test for coronavirus: Spitting into a tube]
“This is exciting,” said Bob Kocher, a physician, venture capitalist and member of California’s testing task force. “The world is plagued by a scarcity of swabs, so having an alternative is a great thing. Also, no patient likes the feeling of having a swab pushed nearly into your brain to collect COVID-19 samples. It is much easier to spit into a tube.”
The Rutgers test is already up and running. The university and RUCDR Infinite Biologics are producing 20,000 tests a day and expect to ramp up to 50,000 a day, largely for use in New Jersey with a doctor’s prescription. Results are analyzed at the university’s clinical genomics laboratory. It’s a small number, but other labs, hospitals, agencies, and universities can buy testing kits from a Utah firm called Spectrum Solutions that has partnered with Rutgers. They can also use Rutgers data to get their own emergency use authorizations.
The Rutgers researchers saw obvious drawbacks to the virus tests built around nasopharyngeal swabs, Brooks said. Scraping genetic materials from the nether reaches of the sinus passage is painful, making it less likely that people would submit and undermining the broad testing programs needed to reopen the economy. And those swabs pose a danger to health-care workers who have to administer them since the procedure frequently causes patients to sneeze or cough on those workers, many of whom lack proper protective gear.
Home testing with saliva could appeal to people who might be too old, too sick, or too scared to venture out for a test. Even children at school or summer camp could spit into a device and get tested, Brooks said.
“We created a scenario to bring the test to the patient,” Brooks said. “That’s the most groundbreaking part of this.”
To test for accuracy and win over the FDA, Rutgers compared the saliva test against the state-of-the-art nasopharyngeal swab in 60 patients in an effort to find early infection. Each patient was tested twice, once with each approach. The results were identical, Brooks said.
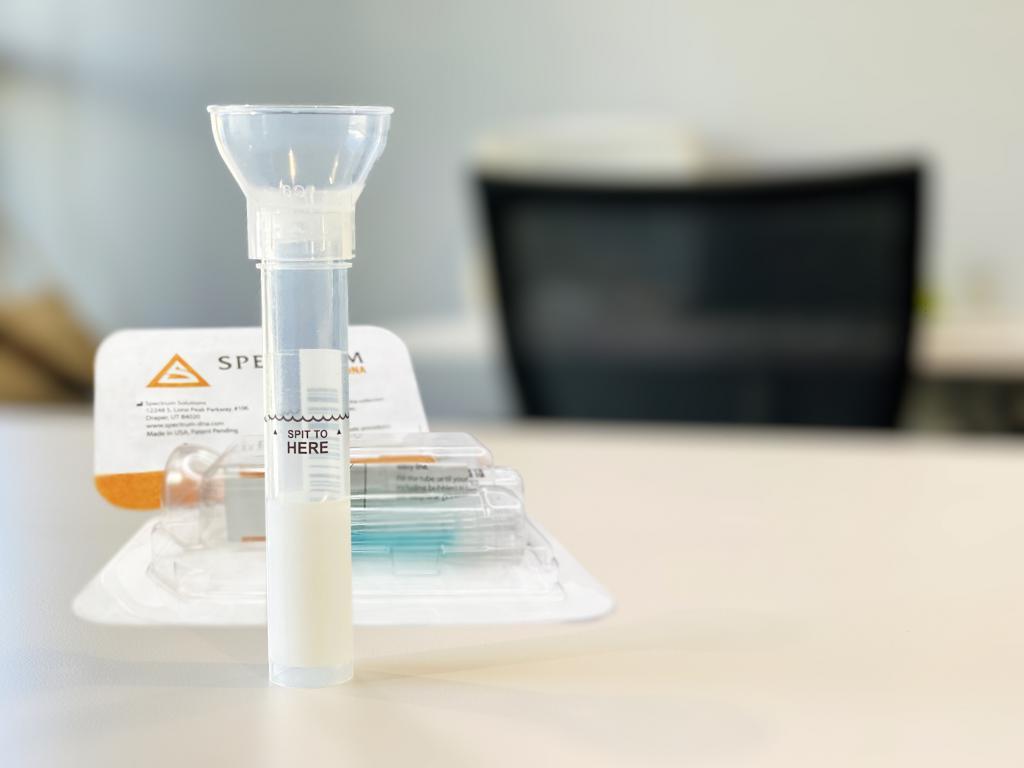
SDNA saliva collection devices at home. ©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Researchers from the University of Colorado at Boulder have launched a firm called Darwin Biosciences and are developing the “SickStick,” a device to measure the presence of the virus in saliva. Oklahoma State University, while awaiting FDA authorization, is using saliva to test thousands of nursing home patients. And in Connecticut, scientists are working on a test strip that could be taken at home for immediate results, without having to ship it to a lab — akin to a home pregnancy test.
[The newest way to test for coronavirus: Spitting into a tube]
“This is exciting,” said Bob Kocher, a physician, venture capitalist and member of California’s testing task force. “The world is plagued by a scarcity of swabs, so having an alternative is a great thing. Also, no patient likes the feeling of having a swab pushed nearly into your brain to collect COVID-19 samples. It is much easier to spit into a tube.”
The Rutgers test is already up and running. The university and RUCDR Infinite Biologics are producing 20,000 tests a day and expect to ramp up to 50,000 a day, largely for use in New Jersey with a doctor’s prescription. Results are analyzed at the university’s clinical genomics laboratory. It’s a small number, but other labs, hospitals, agencies, and universities can buy testing kits from a Utah firm called Spectrum Solutions that has partnered with Rutgers. They can also use Rutgers data to get their own emergency use authorizations.
The Rutgers researchers saw obvious drawbacks to the virus tests built around nasopharyngeal swabs, Brooks said. Scraping genetic materials from the nether reaches of the sinus passage is painful, making it less likely that people would submit and undermining the broad testing programs needed to reopen the economy. And those swabs pose a danger to health-care workers who have to administer them since the procedure frequently causes patients to sneeze or cough on those workers, many of whom lack proper protective gear.
Home testing with saliva could appeal to people who might be too old, too sick, or too scared to venture out for a test. Even children at school or summer camp could spit into a device and get tested, Brooks said.
“We created a scenario to bring the test to the patient,” Brooks said. “That’s the most groundbreaking part of this.”
To test for accuracy and win over the FDA, Rutgers compared the saliva test against the state-of-the-art nasopharyngeal swab in 60 patients in an effort to find early infection. Each patient was tested twice, once with each approach. The results were identical, Brooks said.

SDNA saliva collection devices at home. ©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Other entities working on saliva testing lag behind Rutgers with obtaining Medicare and Medicaid reimbursement
The Yale School of Public Health conducted its own study of 44 inpatients and 98 health-care workers and found that saliva samples taken from just inside the mouth provided greater detection and consistency than the nasal swab approach. The study also concluded there was less variability in results with the self-sample collection of saliva.
Two of the health-care workers had traces of the virus in the saliva test that did not show up in the test based on nasopharyngeal swabs. They were quarantined to avoid infecting others at the hospital. Yale is still awaiting an emergency use authorization from the FDA.
In another advantage, saliva tests bypass the need for one part of the supply chain that has caused so many bottlenecks during the pandemic. The Rutgers patients spit into a 2 milliliter cup that has already been manufactured. A chemical solution in the cup preserves the genetic material of the virus so it can be shipped to a laboratory for analysis.
“We scoured what was already commercially available so as not to create more problems,” Brooks said.
The drawback of saliva testing is that it depends on the untrained patient to administer, but Brooks said the challenges were no greater than in other testing devices.
Other entities working on saliva testing lag behind Rutgers, whose tests, like others, are reimbursed by the Centers for Medicare and Medicaid Services at $100 each. Darwin Biosciences, the start-up firm founded by professors from the University of Colorado at Boulder, has said it needs months to finish work and approvals for what they call the “SickStick.”
“Our device acts at the earliest stage of infection. It knows you’re sick before you do,” said Nicholas Meyerson, a scientist in the department of molecular, cellular and developmental biology and chief executive of Darwin Biosciences. But Meyerson told the university online publication CU Boulder Today that the group needs more time; its device might be helpful if a second wave of infections hits.
In Connecticut, another group called Homodeus has also been working on a saliva test that could be done as easily as a home pregnancy test. Jonathan Rothberg, one of the lead researchers, said the test could be made for a dollar or less and scaled into the millions. The goal would be to have results in half an hour, and an iPhone app would enable patients to analyze the data themselves, and then share that data widely and quickly in the event of a breakout.
“So you can spit into a tube. And get results. No lab. No technician. No expensive machines. No wait. The designer enzymes do all the work,” Rothberg said in an email last month. In a tweet this week, he said the company was still “preparing for validation studies for regulatory agencies.”
Rothberg remained optimistic, though. He recently tweeted that for less than going to the moon, America could go to work. And for less than they spend on 80 billion sodas, Americans could do universal testing.
“We’re looking for geniuses to come up with good solutions,” said Harlan Krumholz, a cardiology professor, and public health expert at Yale medical school. “They all need to be tested rigorously, but quickly. That’s the balance.”
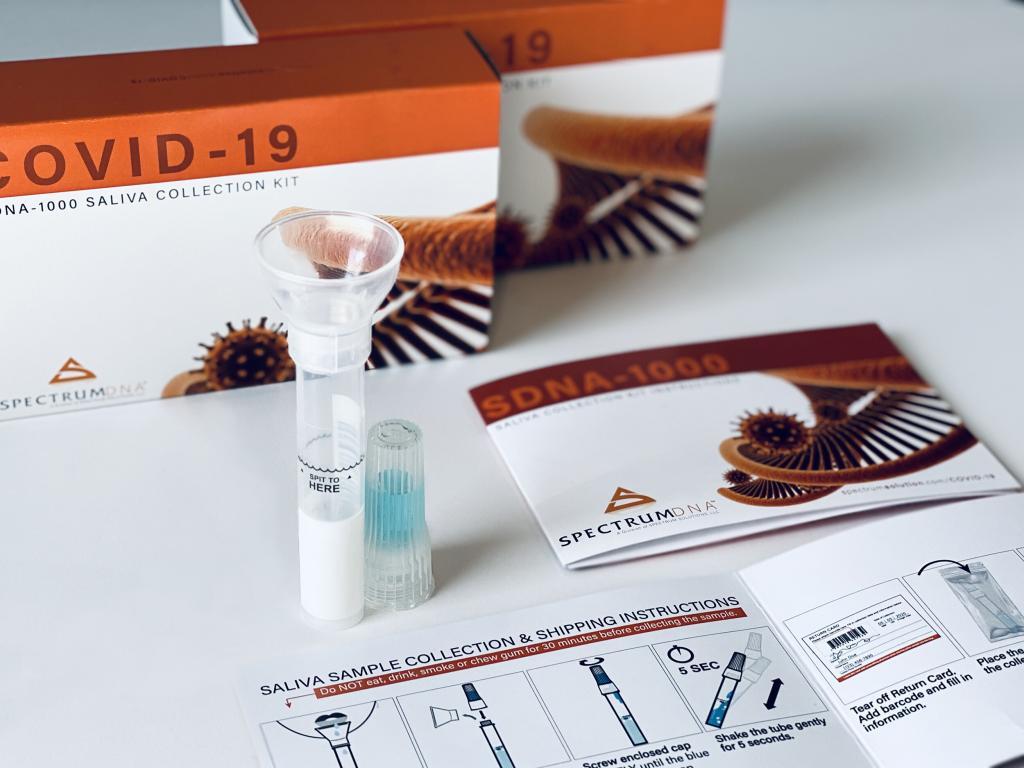
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
The Yale School of Public Health conducted its own study of 44 inpatients and 98 health-care workers and found that saliva samples taken from just inside the mouth provided greater detection and consistency than the nasal swab approach. The study also concluded there was less variability in results with the self-sample collection of saliva.
Two of the health-care workers had traces of the virus in the saliva test that did not show up in the test based on nasopharyngeal swabs. They were quarantined to avoid infecting others at the hospital. Yale is still awaiting an emergency use authorization from the FDA.
In another advantage, saliva tests bypass the need for one part of the supply chain that has caused so many bottlenecks during the pandemic. The Rutgers patients spit into a 2 milliliter cup that has already been manufactured. A chemical solution in the cup preserves the genetic material of the virus so it can be shipped to a laboratory for analysis.
“We scoured what was already commercially available so as not to create more problems,” Brooks said.
The drawback of saliva testing is that it depends on the untrained patient to administer, but Brooks said the challenges were no greater than in other testing devices.
Other entities working on saliva testing lag behind Rutgers, whose tests, like others, are reimbursed by the Centers for Medicare and Medicaid Services at $100 each. Darwin Biosciences, the start-up firm founded by professors from the University of Colorado at Boulder, has said it needs months to finish work and approvals for what they call the “SickStick.”
“Our device acts at the earliest stage of infection. It knows you’re sick before you do,” said Nicholas Meyerson, a scientist in the department of molecular, cellular and developmental biology and chief executive of Darwin Biosciences. But Meyerson told the university online publication CU Boulder Today that the group needs more time; its device might be helpful if a second wave of infections hits.
In Connecticut, another group called Homodeus has also been working on a saliva test that could be done as easily as a home pregnancy test. Jonathan Rothberg, one of the lead researchers, said the test could be made for a dollar or less and scaled into the millions. The goal would be to have results in half an hour, and an iPhone app would enable patients to analyze the data themselves, and then share that data widely and quickly in the event of a breakout.
“So you can spit into a tube. And get results. No lab. No technician. No expensive machines. No wait. The designer enzymes do all the work,” Rothberg said in an email last month. In a tweet this week, he said the company was still “preparing for validation studies for regulatory agencies.”
Rothberg remained optimistic, though. He recently tweeted that for less than going to the moon, America could go to work. And for less than they spend on 80 billion sodas, Americans could do universal testing.
“We’re looking for geniuses to come up with good solutions,” said Harlan Krumholz, a cardiology professor, and public health expert at Yale medical school. “They all need to be tested rigorously, but quickly. That’s the balance.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.


