WALL STREET JOURNAL:
THE NEW YORK TIMES: Just Spit and Wait – New Coronavirus Test Offers Advantage
By Charity L. Scott
Published: June 2, 2020
Published: June 2, 2020
Companies are starting to roll out tests that can diagnose coronavirus infections at home, offering people who are seeking to return to work a potentially safer, more accessible option to check their health.
Yet experts worry about the accuracy of the results generated by the at-home tests, costs that insurers often don’t cover, and other factors that could limit use.
At-home tests are the next wave of coronavirus diagnostics, following tests given by doctors at offices and hospitals. Some of the newest ones use a person’s saliva to detect an infection. All of the tests, whether done at home or not, must be sent to a lab for analysis.
The U.S. Food and Drug Administration has authorized the emergency use of six coronavirus at-home collection kits, with the first at-home test greenlighted April 20.
The tests promise to expand diagnoses to people with disabilities, compromised immune systems or limited access to transportation who have struggled to leave their homes, health experts and industry officials say. Overall, they will add to the number of desperately needed tests available in the U.S.
The market for the tests is large, analysts say. The kits will also appeal to companies looking to test their workers before reopening, as well as colleges and universities that want to test faculty and students, said Brian Tanquilut, health-care services analyst at Jefferies LLC.
“With more than 20 million college students in the country, that’s a fairly sizable number of tests,” Mr. Tanquilut said. “It could be a really big market for that reason.”
Sales of at-home Covid-19 diagnostic tests could reach as high as $816 million this year, estimates Frost & Sullivan analyst Amartya Bose.

Saliva test kit is displayed at Rutgers University’s | CHRISTOPHER GREGORY FOR THE WALL STREET JOURNAL
Companies are starting to roll out tests that can diagnose coronavirus infections at home, offering people who are seeking to return to work a potentially safer, more accessible option to check their health.
Yet experts worry about the accuracy of the results generated by the at-home tests, costs that insurers often don’t cover, and other factors that could limit use.
At-home tests are the next wave of coronavirus diagnostics, following tests given by doctors at offices and hospitals. Some of the newest ones use a person’s saliva to detect an infection. All of the tests, whether done at home or not, must be sent to a lab for analysis.
The U.S. Food and Drug Administration has authorized the emergency use of six coronavirus at-home collection kits, with the first at-home test greenlighted April 20.
The tests promise to expand diagnoses to people with disabilities, compromised immune systems or limited access to transportation who have struggled to leave their homes, health experts and industry officials say. Overall, they will add to the number of desperately needed tests available in the U.S.
The market for the tests is large, analysts say. The kits will also appeal to companies looking to test their workers before reopening, as well as colleges and universities that want to test faculty and students, said Brian Tanquilut, health-care services analyst at Jefferies LLC.
“With more than 20 million college students in the country, that’s a fairly sizable number of tests,” Mr. Tanquilut said. “It could be a really big market for that reason.”
Sales of at-home Covid-19 diagnostic tests could reach as high as $816 million this year, estimates Frost & Sullivan analyst Amartya Bose.

Saliva test kit is displayed at Rutgers University’s | CHRISTOPHER GREGORY FOR THE WALL STREET JOURNAL
Saliva is a much more consistent sample type. Virus was detected in saliva 21% more than nasopharyngeal swabs.
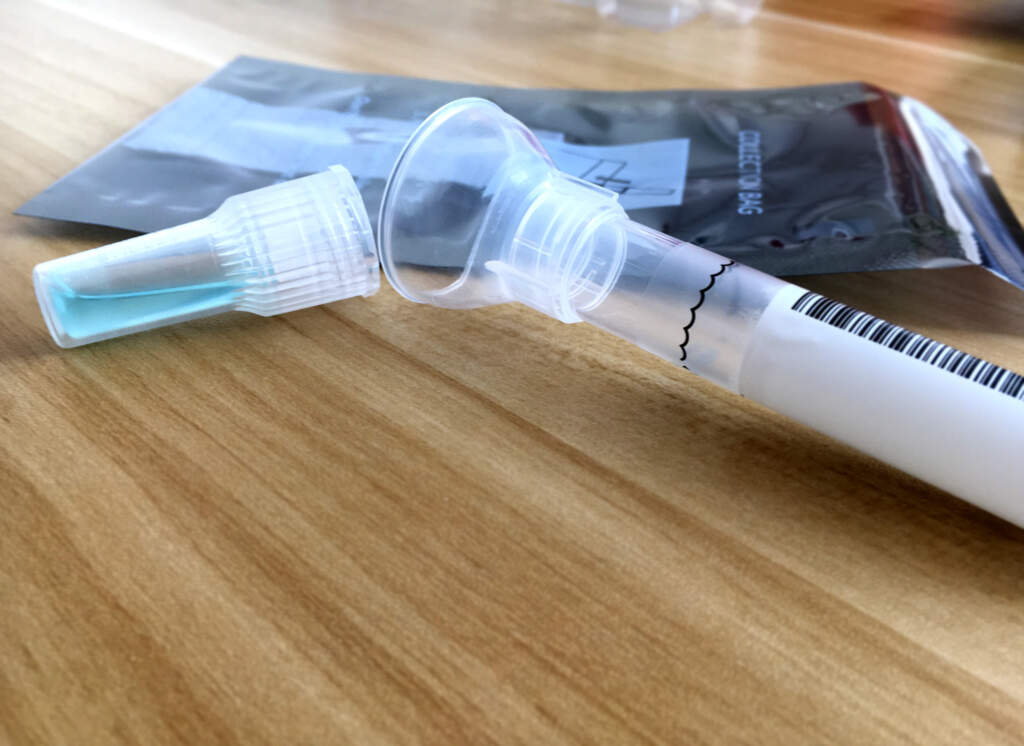
So far, the at-home tests take three different forms. Some rely on samples collected at people’s homes by health workers such as nurses using swabs in the nose and throat, while other tests let people use swabs inside a nostril or spit collected in a test tube.
Saliva tests are appealing as an at-home test, partly because it is easier to spit into a test tube than inserting a swab up the nose, said Andrew Brooks, chief operating officer and director of technology development at Rutgers University’s RUCDR Infinite Biologics, a laboratory that developed a saliva test that won emergency-use authorization May 7.
While the technology behind at-home kits is similar to what other Covid-19 diagnostic tests use, where and when the sample is collected can affect accuracy, said Alan Wells, professor of pathology at the University of Pittsburgh and medical director at UPMC Clinical Laboratories.
A sample taken from saliva or a nose swab could return a false negative, Dr. Wells said, if the disease has moved onto the lungs, as it often does. For this reason, sampling from the nostril or saliva increases the rate of false negatives by 10% to 20%, he said. “I would trust a positive [test result] as being positive,” he added. “If you get a negative, it doesn’t mean it truly is negative.”
Mistakes taking samples can also affect accuracy, said Anne Wyllie, an associate research scientist at Yale School of Public Health. She led a study comparing samples taken from different places in the same patient and found the coronavirus was detected at different rates depending on the site of the sample.
The virus was detected in nasopharyngeal swabs, the samples taken from deep down the throat that is considered the best place to test, but not in saliva from the same patient 8% of the time, according to the report published online April 22 on the preprint server medRxiv. The virus was detected in saliva but not nasopharyngeal swabs 21% of the time.
The study didn’t look at nasal swabs. Other studies have shown that “saliva is a much more consistent sample type,” Dr. Wyllie said.

© Spectrum Solutions™ COVID-19 SDNA-1000 Saliva Collection Testing Kit | Bloomberg News/George Frey
So far, the at-home tests take three different forms. Some rely on samples collected at people’s homes by health workers such as nurses using swabs in the nose and throat, while other tests let people use swabs inside a nostril or spit collected in a test tube.
Saliva tests are appealing as an at-home test, partly because it is easier to spit into a test tube than inserting a swab up the nose, said Andrew Brooks, chief operating officer and director of technology development at Rutgers University’s RUCDR Infinite Biologics, a laboratory that developed a saliva test that won emergency-use authorization May 7.
While the technology behind at-home kits is similar to what other Covid-19 diagnostic tests use, where and when the sample is collected can affect accuracy, said Alan Wells, professor of pathology at the University of Pittsburgh and medical director at UPMC Clinical Laboratories.
A sample taken from saliva or a nose swab could return a false negative, Dr. Wells said, if the disease has moved onto the lungs, as it often does. For this reason, sampling from the nostril or saliva increases the rate of false negatives by 10% to 20%, he said. “I would trust a positive [test result] as being positive,” he added. “If you get a negative, it doesn’t mean it truly is negative.”
Mistakes taking samples can also affect accuracy, said Anne Wyllie, an associate research scientist at Yale School of Public Health. She led a study comparing samples taken from different places in the same patient and found the coronavirus was detected at different rates depending on the site of the sample.
The virus was detected in nasopharyngeal swabs, the samples taken from deep down the throat that is considered the best place to test, but not in saliva from the same patient 8% of the time, according to the report published online April 22 on the preprint server medRxiv. The virus was detected in saliva but not nasopharyngeal swabs 21% of the time.
The study didn’t look at nasal swabs. Other studies have shown that “saliva is a much more consistent sample type,” Dr. Wyllie said.

© Spectrum Solutions™ COVID-19 SDNA-1000 Saliva Collection Testing Kit | Bloomberg News/George Frey
Limiting at-home testing is the capacity of labs to analyze results.
The FDA considers nasopharyngeal swabs to be most accurate, but authorized use of nasal tests because they have relatively comparable performance, an agency spokesman said. The FDA has seen a variable performance with tests using saliva, and has issued specific recommendations for labs looking to validate tests with that sample type, he said.
To address the collection concerns, some test makers are sending trained nurses and other health workers to people’s homes to take throat or nasopharyngeal swabs. Such measures, however, are one limitation on the number of at-home tests available.
Microdrop LLC sends nurses to the homes of Houston residents who are elderly, disabled, or don’t have access to mass transit, said Jani Tuomi, co-founder of the at-home testing company. The nurses wear masks, gloves, and other protective gear.
The Houston city government is paying for the tests, which list for $135, said Mr. Tuomi. The test maker, which can process more than 10,000 swab tests a week, plans to give the tests in Texas and select other areas later, he said.
“I think our focus should be in our own backyard,” Mr. Tuomi said.
Also limiting at-home testing is the capacity of labs to analyze results. Rutgers University’s RUCDR Infinite Biologics can process 30,000 tests a day. Given the constraints and FDA concerns about detecting infections in asymptomatic people, the lab’s marketing partners are restricting sales to those with symptoms, Dr. Brooks said.
One partner, Hims Inc., asks potential customers to fill out an online assessment asking questions including whether they have come into contact with any confirmed cases and if they are experiencing any symptoms associated with Covid-19 like a dry cough or shortness of breath.
“The FDA is trying to prioritize those that are symptomatic, so, if you do not have any symptoms at all, physicians won’t be able to prescribe the test at this time,” Hims Chief Executive Andrew Dudum said.
The FDA considers nasopharyngeal swabs to be most accurate, but authorized use of nasal tests because they have relatively comparable performance, an agency spokesman said. The FDA has seen a variable performance with tests using saliva, and has issued specific recommendations for labs looking to validate tests with that sample type, he said.
To address the collection concerns, some test makers are sending trained nurses and other health workers to people’s homes to take throat or nasopharyngeal swabs. Such measures, however, are one limitation on the number of at-home tests available.
Microdrop LLC sends nurses to the homes of Houston residents who are elderly, disabled, or don’t have access to mass transit, said Jani Tuomi, co-founder of the at-home testing company. The nurses wear masks, gloves, and other protective gear.
The Houston city government is paying for the tests, which list for $135, said Mr. Tuomi. The test maker, which can process more than 10,000 swab tests a week, plans to give the tests in Texas and select other areas later, he said.
“I think our focus should be in our own backyard,” Mr. Tuomi said.
Also limiting at-home testing is the capacity of labs to analyze results. Rutgers University’s RUCDR Infinite Biologics can process 30,000 tests a day. Given the constraints and FDA concerns about detecting infections in asymptomatic people, the lab’s marketing partners are restricting sales to those with symptoms, Dr. Brooks said.
One partner, Hims Inc., asks potential customers to fill out an online assessment asking questions including whether they have come into contact with any confirmed cases and if they are experiencing any symptoms associated with Covid-19 like a dry cough or shortness of breath.
“The FDA is trying to prioritize those that are symptomatic, so, if you do not have any symptoms at all, physicians won’t be able to prescribe the test at this time,” Hims Chief Executive Andrew Dudum said.
Another constraint on access is price.
Another constraint on access is price. The Rutgers tests cost about $150 out-of-pocket, while a test from Everlywell Inc. costs $109 and a test from PrivaPath Diagnostics Inc. is priced at $129. Mr. Dudum said the company is talking to insurance companies to get the full costs of the test covered, but currently patients have received only partial reimbursement.
Health insurers will pay upfront for a nasal-swab test from Laboratory Corp. of America Holdings, which charges $119 to consumers buying the test directly, a company spokeswoman said. For the uninsured, the company will seek government reimbursement, she said.
Mikey Smith, a restaurant manager from Kingwood, Texas, said he wouldn’t have ordered the LabCorp test last month if it wasn’t covered by his insurance, even though he wanted to make sure it was safe to spend time with family over the recent Memorial Day weekend after interacting with strangers.
“$119—I don’t have that to blow every week,” said Mr. Smith, 41 years old, whose test came back negative.
Another constraint on access is price. The Rutgers tests cost about $150 out-of-pocket, while a test from Everlywell Inc. costs $109 and a test from PrivaPath Diagnostics Inc. is priced at $129. Mr. Dudum said the company is talking to insurance companies to get the full costs of the test covered, but currently patients have received only partial reimbursement.
Health insurers will pay upfront for a nasal-swab test from Laboratory Corp. of America Holdings, which charges $119 to consumers buying the test directly, a company spokeswoman said. For the uninsured, the company will seek government reimbursement, she said.
Mikey Smith, a restaurant manager from Kingwood, Texas, said he wouldn’t have ordered the LabCorp test last month if it wasn’t covered by his insurance, even though he wanted to make sure it was safe to spend time with family over the recent Memorial Day weekend after interacting with strangers.
“$119—I don’t have that to blow every week,” said Mr. Smith, 41 years old, whose test came back negative.

Spectrum
in the News
Spectrum
in the News
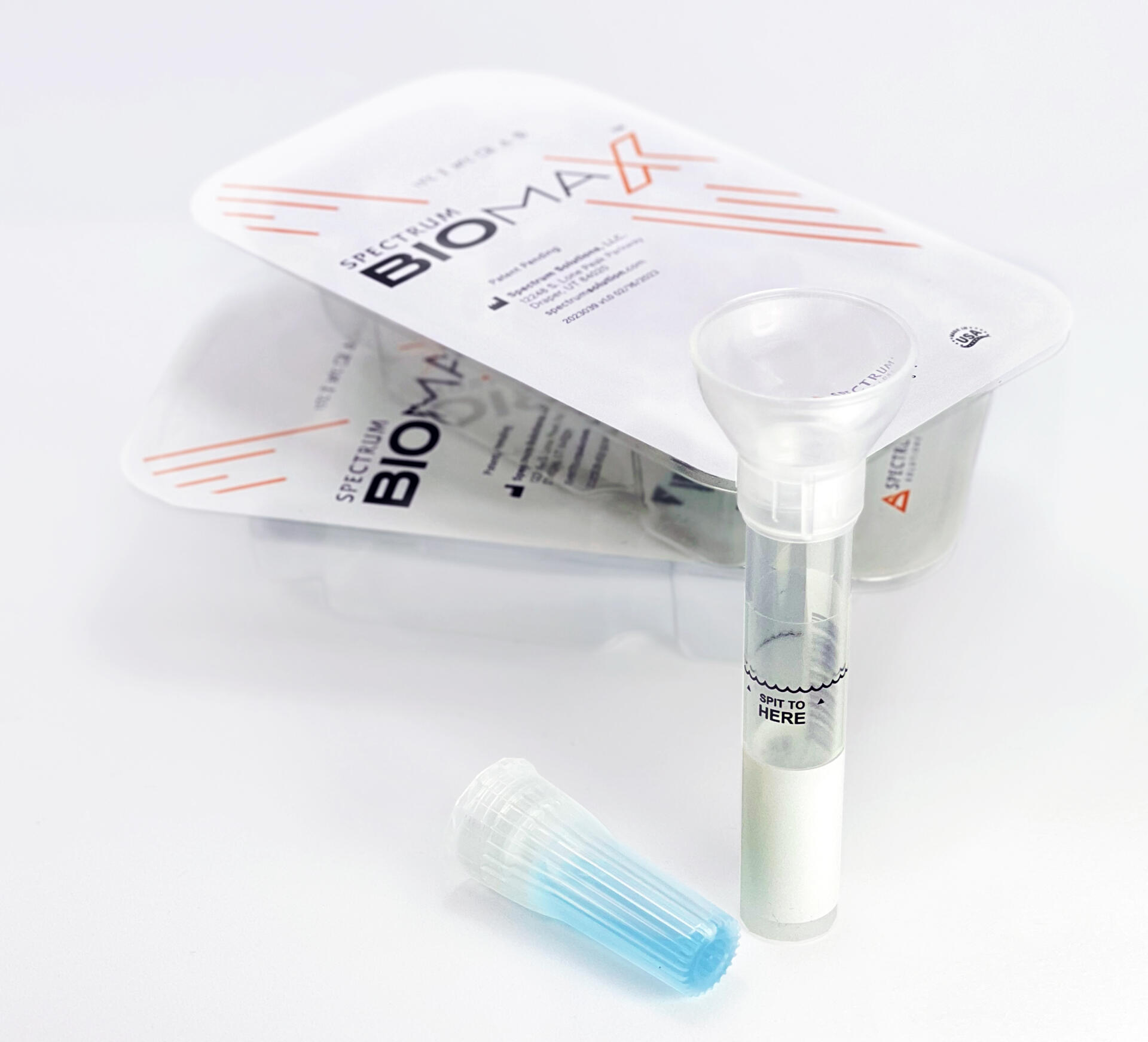
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.


