ABC4 Special Report:
Utah-Based Spectrum Solutions Saliva Collection Device Used in new COVID-19 Testing
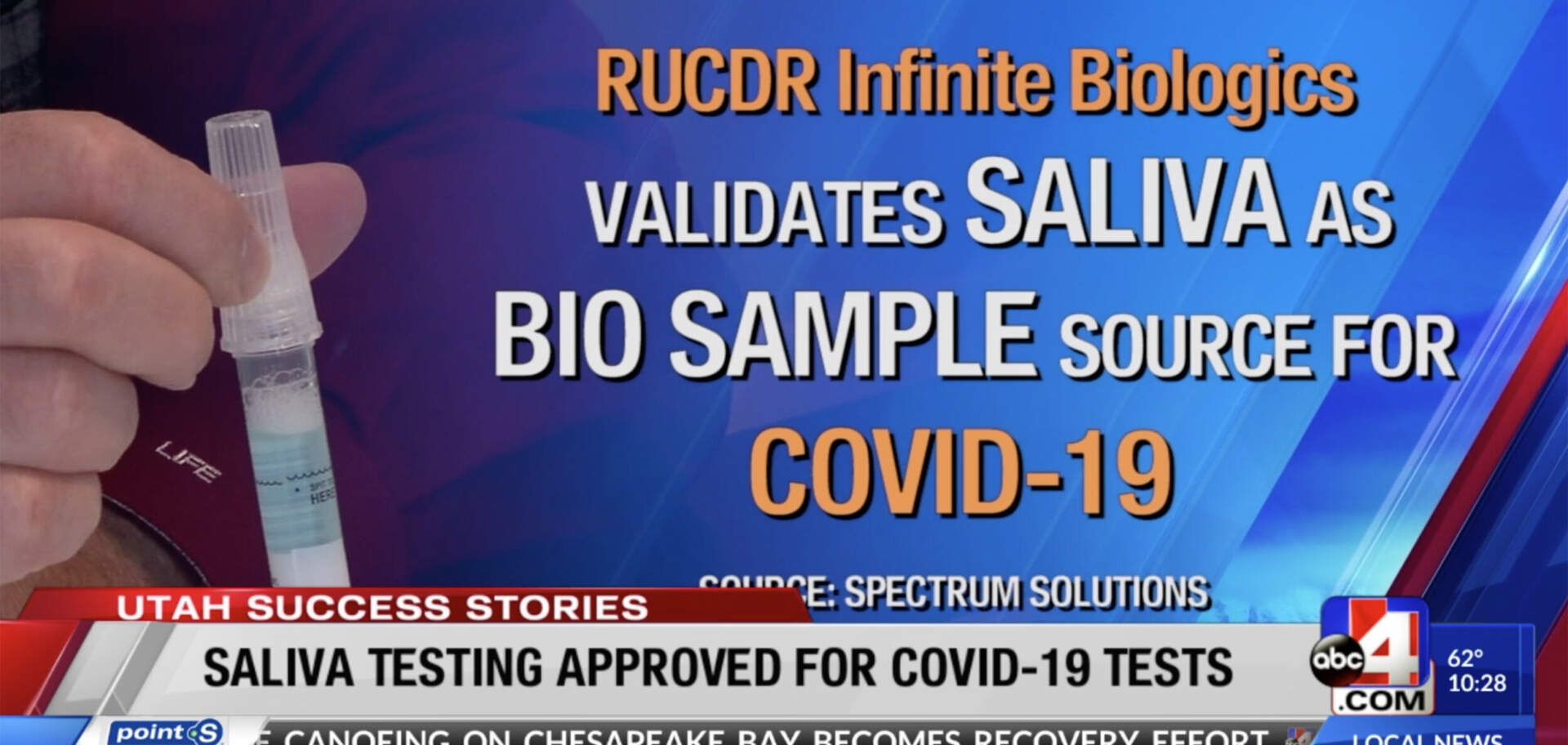
It was just announced that researchers from RUCDR Infinite Biologics have successfully validated saliva as being a viable bio sample source for COVID-19 using the Spectrum DNA saliva collection device.
by: Douglas Jessop
Posted: Updated:
(ABC4 NEWS – DRAPER, UT) Currently COVID19 tests are being done with the use of a long swab known as a nasal phalangeal swab. The swab goes up into the nasal cavity and into the sinuses. One of the problems getting more people tested is a shortage of those swabs.
It was just announced that researchers from RUCDR Infinite Biologics have successfully validated saliva as being a viable biosample source for COVID-19.
Utah-based Spectrum Solutions was the sole saliva collection device used in that study.
Chief Operating Officer, Bill Phillips explains; “We had to do comparable studies to the swab. We collected over 75 samples of swab and saliva devices with people that do and do not have COVID 19.” I asked Bill what the results were. He quickly replied; “100 percent comparable.”
You may have seen or even used their DNA tests they make for companies like Ancestry.com. Their patented collection system works the same for collecting saliva for COVID19 testing.
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.