Saliva Testing Innovation Brings MLB Back to the Game
CASE STUDY
Leslie Titus Bryant
JAN 05, 2022
THE CHALLENGE:
In the wake of the COVID-19 2020 global pandemic, the world’s economy slowed to a crawl, office buildings were empty, students stayed home from school, stadiums were eerily silent, and we all had more questions than available answers.
While America tried to grapple with the “new normal” of stay-at-home orders and social distancing, healthcare organizations and governments began to do what they could to help stem the tide. Testing for the virus was a priority and it immediately became shockingly clear to the world that shortages in testing supplies and PPE equipment were causing a very real global threat to finding answers and providing treatment for this new virus. The only testing option that was available at the time involved nasal swabs, but within the first few weeks of the pandemic, the swabs were in critically short supply or simply out of stock.
As time went on players and fans of Major League Baseball (MLB™) held their breath awaiting word of whether the unthinkable would happen and there would be no baseball right when people needed it most. This sentiment was echoed in the press with USA Today lamenting, “The MLB already missed its planned opening day, and it is unclear when baseball could start.” And SportingNews asked, “Will MLB Cancel the 2020 Season?”
A major element that clouded the future of the 2020 season was the very diagnostic tests themselves. The nature of the swabs required medical professionals to insert the swab into a person’s nasal cavity to collect a biosample. This process is almost always painful and as more people became aware of the test, it became a source of anxiety and stress, which stopped many people from deciding to get themselves or their loved ones tested.
The league and club owners were understandably reluctant to inflict pain on their athletes and staff on a regular basis. Unless something changed, the 2020 season was doomed.
Saliva Testing Innovation Brings MLB Back to the Game
CASE STUDY
Leslie Titus Bryant
JAN 05, 2022
THE CHALLENGE:
In the wake of the COVID-19 2020 global pandemic, the world’s economy slowed to a crawl, office buildings were empty, students stayed home from school, stadiums were eerily silent, and we all had more questions than available answers.
While America tried to grapple with the “new normal” of stay-at-home orders and social distancing, healthcare organizations and governments began to do what they could to help stem the tide. Testing for the virus was a priority and it immediately became shockingly clear to the world that shortages in testing supplies and PPE equipment were causing a very real global threat to finding answers and providing treatment for this new virus. The only testing option that was available at the time involved nasal swabs, but within the first few weeks of the pandemic, the swabs were in critically short supply or simply out of stock.
As time went on players and fans of Major League Baseball (MLB™) held their breath awaiting word of whether the unthinkable would happen and there would be no baseball right when people needed it most. This sentiment was echoed in the press with USA Today lamenting, “The MLB already missed its planned opening day, and it is unclear when baseball could start.” And SportingNews asked, “Will MLB Cancel the 2020 Season?”
A major element that clouded the future of the 2020 season was the very diagnostic tests themselves. The nature of the swabs required medical professionals to insert the swab into a person’s nasal cavity to collect a biosample. This process is almost always painful and as more people became aware of the test, it became a source of anxiety and stress, which stopped many people from deciding to get themselves or their loved ones tested.
The league and club owners were understandably reluctant to inflict pain on their athletes and staff on a regular basis. Unless something changed, the 2020 season was doomed.
The Only Spitting Allowed in
2020 will Bring Baseball Back
THE SOLUTION:
That’s when the team at Spectrum Solutions asked the question “How can we be part of the solution?” This launched a chain of events that led to some groundbreaking scientific innovations. The company had previously designed a saliva collection device for at-home DNA tests for consumers to learn about their genetic lineage. The team took on the challenge to discover whether saliva could be used to test for COVID-19. They worked hand-in-hand with researchers from Rutgers University to test whether saliva could indeed be a viable bio-sample.
As it turns out, when researchers compared saliva to traditional swab-based COVID-19 testing methods, saliva proved to actually be more sensitive, trigger far fewer false negatives (which is a significant issue with swabs), AND delivered an increase in testing accuracy. Independent studies soon followed from universities and researchers across the nation and around the world. Reports echoed support for the remarkable solution and validated that saliva collected and preserved with Spectrum’s SDNA-1000 for detecting COVID-19 infections was viable. All of these institutions used the SDNA-1000 because of three distinct and unique properties of the company’s patented blue preservation solution that is used with the device:
- The proprietary blue preservation solution neutralizes the active virus. Without that capability, saliva samples with the live virus would put lab workers at extreme risk of exposure.
- The solution preserves and stabilizes the fragile RNA, which is what is used to detect and test for the virus.
- The solution stabilizes the bio-sample even in extreme temperatures and conditions, which allows for shipping around the country (and the world).
The FDA quickly provided the very first Emergency Use Authorization for COVID-19 diagnostic testing using saliva—and specified that because of its unique properties, saliva must exclusively be collected and preserved using the SDNA-1000 saliva collection device.
Ongoing research and news of saliva’s dramatic rise in diagnostic importance flooded the media and this relatively small, privately-held Utah company repeatedly found itself in headlines from around the world.
Leaders at MLB had three key requirements when it came to COVID-19 testing:
- COVID-19 tests had to be painless
- The tests had to be easy to use
- The tests had to give the option to be self-administered
- The supply of tests needed to be adequate to meet their demands with ease
The Spectrum Solutions SDNA-1000 saliva collection device easily met these requirements with flying colors.
In August, it became official with a partnership announcement. Major League Baseball named Spectrum Solutions, LLC as a partner in its COVID-19 testing program for the 2020 season. Spectrum Solutions supplied MLB and its Clubs with its SDNA-1000 saliva collection kits for all player and staff testing. The non-invasive saliva collection device has become a critical component of MLB’s comprehensive COVID-19 health monitoring and testing plan and served as a key safeguard to help maintain the health and safety of MLB personnel during the pandemic.
“We would like to thank Spectrum Solutions for playing such an integral role in Major League Baseball’s return to the field in 2020. We needed a safe, reliable, and non-invasive way to regularly test and monitor players and Club personnel this season. Spectrum has proven to be the best option available for our ongoing needs. We appreciate Spectrum’s support, collaboration, and assistance during this challenging time.”
Jon Coyles: VP, Health & Safety for MLB
The Only Spitting Allowed in
2020 will Bring Baseball Back
THE SOLUTION:
That’s when the team at Spectrum Solutions asked the question “How can we be part of the solution?” This launched a chain of events that led to some groundbreaking scientific innovations. The company had previously designed a saliva collection device for at-home DNA tests for consumers to learn about their genetic lineage. The team took on the challenge to discover whether saliva could be used to test for COVID-19. They worked hand-in-hand with researchers from Rutgers University to test whether saliva could indeed be a viable bio-sample.
As it turns out, when researchers compared saliva to traditional swab-based COVID-19 testing methods, saliva proved to actually be more sensitive, trigger far fewer false negatives (which is a significant issue with swabs), AND delivered an increase in testing accuracy. Independent studies soon followed from universities and researchers across the nation and around the world. Reports echoed support for the remarkable solution and validated that saliva collected and preserved with Spectrum’s SDNA-1000 for detecting COVID-19 infections was viable. All of these institutions used the SDNA-1000 because of three distinct and unique properties of the company’s patented blue preservation solution that is used with the device:
- The proprietary blue preservation solution neutralizes the active virus. Without that capability, saliva samples with the live virus would put lab workers at extreme risk of exposure.
- The solution preserves and stabilizes the fragile RNA, which is what is used to detect and test for the virus.
- The solution stabilizes the bio-sample even in extreme temperatures and conditions, which allows for shipping around the country (and the world).
The FDA quickly provided the very first Emergency Use Authorization for COVID-19 diagnostic testing using saliva—and specified that because of its unique properties, saliva must exclusively be collected and preserved using the SDNA-1000 saliva collection device.
Ongoing research and news of saliva’s dramatic rise in diagnostic importance flooded the media and this relatively small, privately-held Utah company repeatedly found itself in headlines from around the world.
Leaders at MLB had three key requirements when it came to COVID-19 testing:
- COVID-19 tests had to be painless
- The tests had to be easy to use
- The tests had to give the option to be self-administered
- The supply of tests needed to be adequate to meet their demands with ease
The Spectrum Solutions SDNA-1000 saliva collection device easily met these requirements with flying colors.
In August, it became official with a partnership announcement. Major League Baseball named Spectrum Solutions, LLC as a partner in its COVID-19 testing program for the 2020 season. Spectrum Solutions supplied MLB and its Clubs with its SDNA-1000 saliva collection kits for all player and staff testing. The non-invasive saliva collection device has become a critical component of MLB’s comprehensive COVID-19 health monitoring and testing plan and served as a key safeguard to help maintain the health and safety of MLB personnel during the pandemic.
“We would like to thank Spectrum Solutions for playing such an integral role in Major League Baseball’s return to the field in 2020. We needed a safe, reliable, and non-invasive way to regularly test and monitor players and Club personnel this season. Spectrum has proven to be the best option available for our ongoing needs. We appreciate Spectrum’s support, collaboration, and assistance during this challenging time.”
Jon Coyles: VP, Health & Safety for MLB
How you Collect, Preserve, & Transport Saliva is Pivotal
THE RESULTS
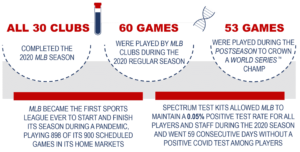
Because of the SDNA saliva collection kits, MLB became the first sports league ever to start and finish its season during a pandemic, playing 898 of its 900 scheduled games and all 30 clubs completed the 2020 MLB Season!
- More than 200,000 SDNA saliva collection kits were used by MLB players, coaches, club staff, league executives, support personnel, and family members to complete the 2020 season
- 12,000 test kits per week were distributed for monitoring purposes throughout the MLB 2020 Summer Camp, Regular Season and Postseason™
- Thousands of free test kits were provided to schools, youth organizations, and healthcare providers within local communities throughout the 2020 MLB Regular Season and 2020-2021 MLB Offseason
- Spectrum test kits allowed MLB to maintain a 0.05% positive test rate for all players and staff during the 2020 season and went 59 consecutive days without a positive COVID test among players.
“The entire team here at Spectrum loves Major League Baseball and we couldn’t be prouder to be part of the solution in helping the league with its key testing needs,” said Bill Phillips, COO of Spectrum Solutions. “Easy to use, pain-free saliva collection for COVID-19 testing is an essential part of MLB’s health and safety protocols. Spectrum is happy our SDNA-1000 kits are serving to support MLB play this 2020 season.”

How you Collect, Preserve, & Transport Saliva is Pivotal
THE RESULTS
Because of the SDNA saliva collection kits, MLB became the first sports league ever to start and finish its season during a pandemic, playing 898 of its 900 scheduled games and all 30 clubs completed the 2020 MLB Season!
- More than 200,000 SDNA saliva collection kits were used by MLB players, coaches, club staff, league executives, support personnel, and family members to complete the 2020 season
- 12,000 test kits per week were distributed for monitoring purposes throughout the MLB 2020 Summer Camp, Regular Season and Postseason™
- Thousands of free test kits were provided to schools, youth organizations, and healthcare providers within local communities throughout the 2020 MLB Regular Season and 2020-2021 MLB Offseason
- Spectrum test kits allowed MLB to maintain a 0.05% positive test rate for all players and staff during the 2020 season and went 59 consecutive days without a positive COVID test among players.
“The entire team here at Spectrum loves Major League Baseball and we couldn’t be prouder to be part of the solution in helping the league with its key testing needs,” said Bill Phillips, COO of Spectrum Solutions. “Easy to use, pain-free saliva collection for COVID-19 testing is an essential part of MLB’s health and safety protocols. Spectrum is happy our SDNA-1000 kits are serving to support MLB play this 2020 season.”



Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask the Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com




