GENOME WEB
Rutgers Gets FDA EUA for Modified Thermo Fisher Coronavirus Assay for Saliva Samples Exclusively Collected With Spectrum’s SDNA-1000
Staff Reporter
Published: April 13, 2020
Published: April 13, 2020
EUA granted to use saliva collection for COVID-19 testing
NEW YORK – The US Food and Drug Administration on Friday granted Emergency Use Authorization to the Rutgers Clinical Genomics Laboratory for a SARS-CoV-2 test originally developed by Thermo Fisher Scientific that is modified for use on additional specimen types, including saliva, as well as with alternative nucleic acid extraction and amplification systems.
The Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is a modified version of the previously authorized Thermo Fisher Applied Biosystems TaqPath COVID-19 Combo Kit for the detection of specific genomic regions of the SARS-CoV-2 nucleocapsid gene, spike gene, and ORF1ab region in nasopharyngeal swab, nasopharyngeal aspirate, and bronchoalveolar lavage specimens.
The Thermo Fisher kit can be run on the Applied Biosystems 7500 Fast Dx Real-Time PCR instrument or other authorized instruments, and later received expanded EUA for manual sample extraction using the MagMax viral/pathogen nucleic acid isolation kit, the Applied Biosystems 7500 Fast real-time PCR system that utilizes DCS versions 1.5.1 and 2.3, and the Applied Biosystems COVID-19 interpretative software v1.1.
The modified kit is authorized for use with oropharyngeal swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab, and saliva specimens. RNA extraction for all specimen types is performed using the PerkinElmer Chemagic 360 automated specimen processing system with the Chemagic Viral DNA/RNA 300 Kit H96. The test runs on the Thermo Fisher Applied Biosystems QuantStudio 5 Real-Time PCR System equipped with software v1.3 or the Applied Biosystems ViiA7 Real-Time PCR System with the Applied Biosystems QuantStudio 5 software v1.3.
“The impact of this approval is significant,” said Andrew Brooks, chief operating officer and director of technology development at RUCDR, who is also a professor in the School of Arts and Sciences Department of Genetics at Rutgers University–New Brunswick, in a statement. “It means we no longer have to put healthcare professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections. We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as self-collection of saliva is more quick and scalable than swab collections. All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
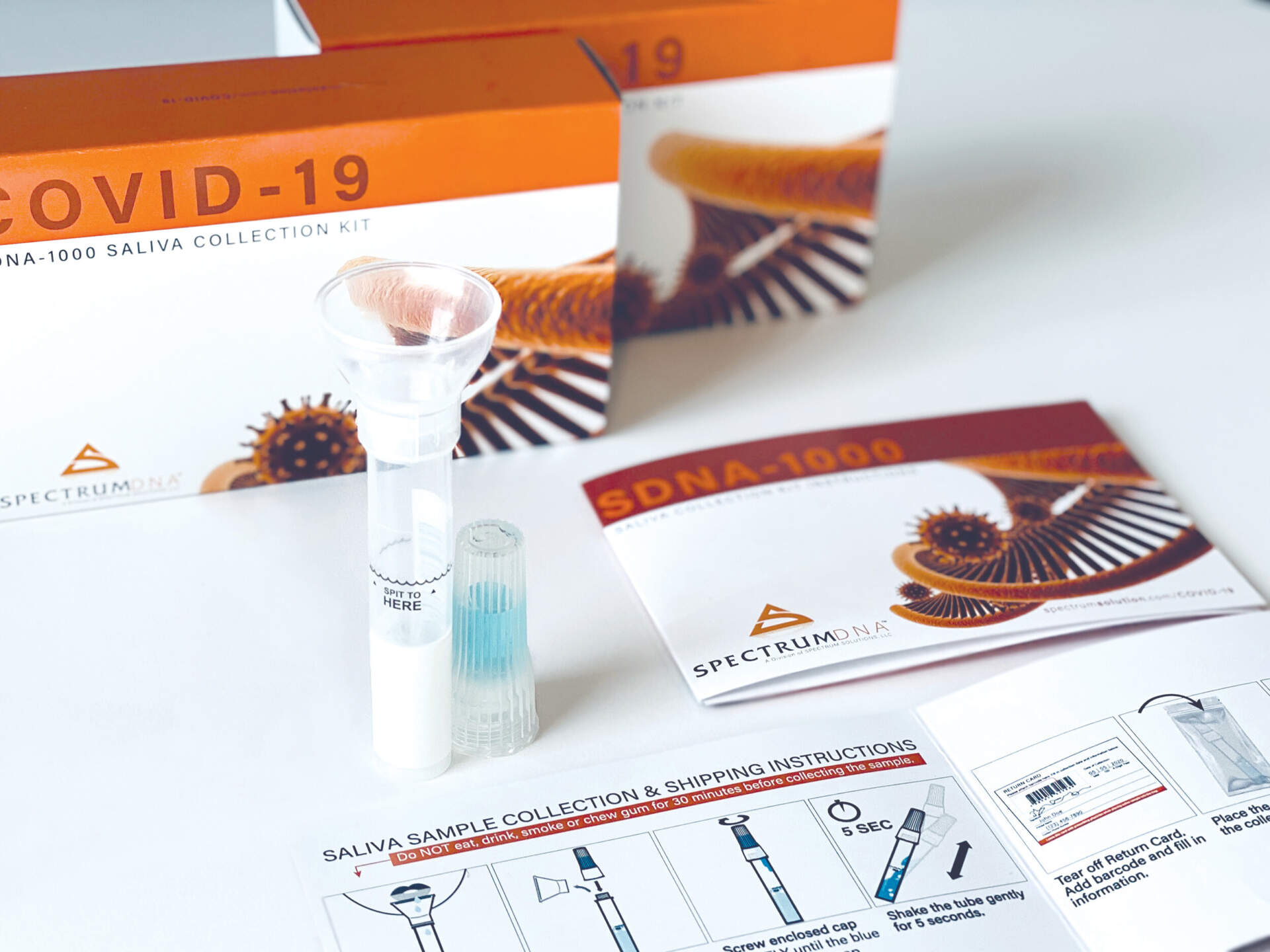
© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
NEW YORK – The US Food and Drug Administration on Friday granted Emergency Use Authorization to the Rutgers Clinical Genomics Laboratory for a SARS-CoV-2 test originally developed by Thermo Fisher Scientific that is modified for use on additional specimen types, including saliva, as well as with alternative nucleic acid extraction and amplification systems.
The Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is a modified version of the previously authorized Thermo Fisher Applied Biosystems TaqPath COVID-19 Combo Kit for the detection of specific genomic regions of the SARS-CoV-2 nucleocapsid gene, spike gene, and ORF1ab region in nasopharyngeal swab, nasopharyngeal aspirate, and bronchoalveolar lavage specimens.
The Thermo Fisher kit can be run on the Applied Biosystems 7500 Fast Dx Real-Time PCR instrument or other authorized instruments, and later received expanded EUA for manual sample extraction using the MagMax viral/pathogen nucleic acid isolation kit, the Applied Biosystems 7500 Fast real-time PCR system that utilizes DCS versions 1.5.1 and 2.3, and the Applied Biosystems COVID-19 interpretative software v1.1.
The modified kit is authorized for use with oropharyngeal swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab, and saliva specimens. RNA extraction for all specimen types is performed using the PerkinElmer Chemagic 360 automated specimen processing system with the Chemagic Viral DNA/RNA 300 Kit H96. The test runs on the Thermo Fisher Applied Biosystems QuantStudio 5 Real-Time PCR System equipped with software v1.3 or the Applied Biosystems ViiA7 Real-Time PCR System with the Applied Biosystems QuantStudio 5 software v1.3.
“The impact of this approval is significant,” said Andrew Brooks, chief operating officer and director of technology development at RUCDR, who is also a professor in the School of Arts and Sciences Department of Genetics at Rutgers University–New Brunswick, in a statement. “It means we no longer have to put healthcare professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections. We can preserve precious personal protective equipment for use in patient care instead of testing. We can significantly increase the number of people tested each and every day as self-collection of saliva is more quick and scalable than swab collections. All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.