AMERICAN SECURITY TODAY:
New COVID-19 Saliva Test Could be Game Changer, Limiting Exposure
By Tammy Waitt
April 15, 2020
Rutgers’ RUCDR Infinite Biologics and its collaborators has been granted emergency use authorization (EUA) by the Food and Drug Administration (FDA), for a new collection approach that utilizes saliva as the primary test biomaterial for the SARS-CoV-2 coronavirus, the first such authorization granted by the federal agency.
This new saliva collection method was developed by RUCDR in partnership with Spectrum Solutions and Accurate Diagnostic Labs (ADL) and will allow for broader population screening than the current method of nose and throat swabs.
“Using saliva to test for COVID-19 overcomes many of the challenges the nation faces that are inherent to current testing methods including supply shortages and risks to exposure for healthcare professionals,” said Stephen Fanning, CEO of Spectrum Solutions.
“The Spectrum Solutions SDNA-1000 Saliva Collection Device was chosen to collect, transport and store saliva specimens for the TaqPath SARS-CoV-2 Assay proof-point study given RUCDR’s experience with Spectrum products across a wide range of applications currently employed,” explained Andrew Brooks, chief operating officer and director of technology development at RUCDR, who also is a professor in the School of Arts and Sciences Department of Genetics at Rutgers University-New Brunswick.“Now, under a medical professional’s direction, the saliva collection can be self-administered by individuals who may be in quarantine or self-isolation, removing the need to be in close contact with medical staff.”
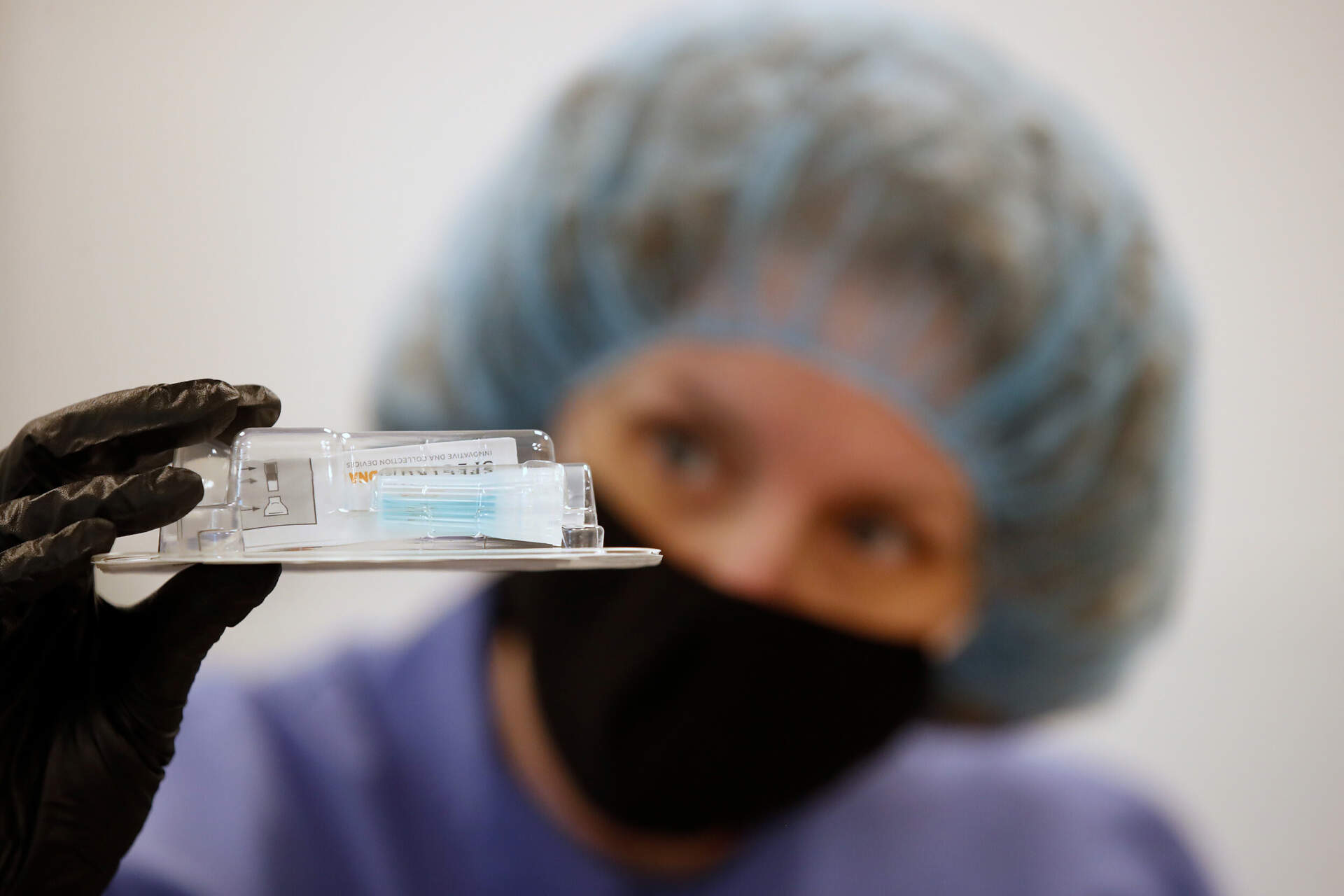
© Spectrum Solutions™ Bloomberg News/George Frey
Rutgers’ RUCDR Infinite Biologics and its collaborators has been granted emergency use authorization (EUA) by the Food and Drug Administration (FDA), for a new collection approach that utilizes saliva as the primary test biomaterial for the SARS-CoV-2 coronavirus, the first such authorization granted by the federal agency.
This new saliva collection method was developed by RUCDR in partnership with Spectrum Solutions and Accurate Diagnostic Labs (ADL) and will allow for broader population screening than the current method of nose and throat swabs.
“Using saliva to test for COVID-19 overcomes many of the challenges the nation faces that are inherent to current testing methods including supply shortages and risks to exposure for healthcare professionals,” said Stephen Fanning, CEO of Spectrum Solutions.
“The Spectrum Solutions SDNA-1000 Saliva Collection Device was chosen to collect, transport and store saliva specimens for the TaqPath SARS-CoV-2 Assay proof-point study given RUCDR’s experience with Spectrum products across a wide range of applications currently employed,” explained Andrew Brooks, chief operating officer and director of technology development at RUCDR, who also is a professor in the School of Arts and Sciences Department of Genetics at Rutgers University-New Brunswick.“Now, under a medical professional’s direction, the saliva collection can be self-administered by individuals who may be in quarantine or self-isolation, removing the need to be in close contact with medical staff.”

© Spectrum Solutions™ Bloomberg News/George Frey
New saliva collection method developed by RUCDR finds value in using Spectrum's SDNA-1000 saliva collection device
“The preservation solution was the main focus of the study to ensure that the virus would be inactivated to the point that we would be able to maintain and stabilize the RNA transcripts for sensitive and specific QPCR analysis in order to determine whether a person has the virus.”
“The device’s intuitive ease-of-use to facilitate minimally supervised or in most cases, complete self-collection is a tremendous advance to current COVID-19 sample collection strategies. The impact of this authorization is significant,” added Brooks.“Together, these benefits will significantly add to expanding access to critical testing needs. It means we no longer have to put health care professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections.”

© Spectrum Solutions™ SDNA-1000 Only EUA Authorized Saliva Collection Device for COVID-19 Testing | Photo Credit: Leslie Titus Bryant
“The preservation solution was the main focus of the study to ensure that the virus would be inactivated to the point that we would be able to maintain and stabilize the RNA transcripts for sensitive and specific QPCR analysis in order to determine whether a person has the virus.”
“The device’s intuitive ease-of-use to facilitate minimally supervised or in most cases, complete self-collection is a tremendous advance to current COVID-19 sample collection strategies. The impact of this authorization is significant,” added Brooks.“Together, these benefits will significantly add to expanding access to critical testing needs. It means we no longer have to put health care professionals at risk for infection by performing nasopharyngeal or oropharyngeal collections.”

© Spectrum Solutions™ SDNA-1000 Only EUA Authorized Saliva Collection Device for COVID-19 Testing | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.