PRESS RELEASE SLC:
THE NEW YORK TIMES: Just Spit and Wait – New Coronavirus Test Offers Advantage
Expanded Authorization for at-home saliva collection granted exclusively using Spectrum’s SDNA-1000
Leslie Titus Bryant
Spectrum Solutions Corporate Spokesperson,
Director of Marketing & Brand
Spectrum Solutions Corporate Spokesperson,
Director of Marketing & Brand
Spectrum’s patented saliva preservation chemistry successfully demonstrated its unique ability to protect COVID-19 transcripts for extended periods making at-home sample collection and transport to Clinical Laboratory Improvement Amendments (CLIA) testing labs a reality.
SALT LAKE CITY–(BUSINESS WIRE)–Spectrum Solutions today announced the U.S. Food and Drug Administration (FDA) expanded the existing EUA200090 awarded to the Rutgers Clinical Genomics Laboratory to now include the at-home collection of saliva samples for COVID-19 testing. The basis for extending the device’s scope of authorization was to include delivery of Spectrum’s SDNA-1000 saliva collection device direct-to-patients at home for self-collection of saliva testing samples. The FDA’s expanded scope of authorization focused on the SDNA-1000 and its proven RNA preservation ability as well as the patient’s ease-of-use. This marks the first, and currently only, authorization by the FDA approving doctor prescribed COVID-19 testing allowing at-home saliva collection.
“Spectrum engineered its SDNA-1000 saliva collection device to eliminate common user collection errors in a universally intuitive, 3-step process which provides visual confirmation when successfully completed”
“The FDA recognized that current methods of obtaining biosamples for COVID-19 testing were limited in scope and scale due to supply shortages and the current testing methods were also constantly putting healthcare workers at risk of exposure. We’ve developed a better, more innovative solution,” said Stephen Fanning, CEO at Spectrum Solutions. “We are excited to announce that after extensive safety testing and validation, the FDA has additionally granted the SDNA-1000 authorization for prescribed use in at-home testing for COVID-19. This new exclusive authorization for direct-to-patient delivery using the SDNA-1000 saliva collection kit brings immediate relief to healthcare facilities while expanding their ability to easily test and help more people in the comfort and safety of their homes.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum’s patented saliva preservation chemistry successfully demonstrated its unique ability to protect COVID-19 transcripts for extended periods making at-home sample collection and transport to Clinical Laboratory Improvement Amendments (CLIA) testing labs a reality.
SALT LAKE CITY–(BUSINESS WIRE)–Spectrum Solutions today announced the U.S. Food and Drug Administration (FDA) expanded the existing EUA200090 awarded to the Rutgers Clinical Genomics Laboratory to now include the at-home collection of saliva samples for COVID-19 testing. The basis for extending the device’s scope of authorization was to include delivery of Spectrum’s SDNA-1000 saliva collection device direct-to-patients at home for self-collection of saliva testing samples. The FDA’s expanded scope of authorization focused on the SDNA-1000 and its proven RNA preservation ability as well as the patient’s ease-of-use. This marks the first, and currently, only authorization by the FDA approving doctor prescribed COVID-19 testing allowing at-home saliva collection.
“Spectrum engineered its SDNA-1000 saliva collection device to eliminate common user collection errors in a universally intuitive, 3-step process which provides visual confirmation when successfully completed”
“The FDA recognized that current methods of obtaining biosamples for COVID-19 testing were limited in scope and scale due to supply shortages and the current testing methods were also constantly putting healthcare workers at risk of exposure. We’ve developed a better, more innovative solution,” said Stephen Fanning, CEO at Spectrum Solutions. “We are excited to announce that after extensive safety testing and validation, the FDA has additionally granted the SDNA-1000 authorization for prescribed use in at-home testing for COVID-19. This new exclusive authorization for direct-to-patient delivery using the SDNA-1000 saliva collection kit brings immediate relief to healthcare facilities while expanding their ability to easily test and help more people in the comfort and safety of their homes.”

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Innovative engineering and a huge displays of driven creativity are continuing to prove greatness can actually keep getting greater.
“Spectrum engineered its SDNA-1000 saliva collection device to eliminate common user collection errors in a universally intuitive, 3-step process which provides visual confirmation when successfully completed,” said Jeremy Johnson, research and development engineer at Spectrum Solutions. “We not only engineered this device to make self-collection intuitive, but we also added a preservation chemistry that has proven itself to be the catalyst needed to deliver to labs the quality samples required for accurate COVID-19 detection.”
How It Works
- Individuals looking to purchase this EUA authorized saliva-based at home COVID-19 test should contact their doctor to order or visit spectrumsolution.com/COVID19-at-home for an authorized seller list.
- Patient arranges an appointment with a physician, which could be in person at the doctor’s office or via a telemedicine session.
- If physician determines the patient is a candidate for a COVID-19 test, the physician will prescribe the test and arrange for the test to be delivered to the patient.
- The patient will follow the simple saliva collection instructions in the kit on how to supply their saliva sample and how to return the sample to the processing lab through direct mail or drop off service.
- The lab will run the sample and report the results to the physician. The physician will then go over the results with the patient and determine the next course of action.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
“Spectrum engineered its SDNA-1000 saliva collection device to eliminate common user collection errors in a universally intuitive, 3-step process which provides visual confirmation when successfully completed,” said Jeremy Johnson, research and development engineer at Spectrum Solutions. “We not only engineered this device to make self-collection intuitive, but we also added a preservation chemistry that has proven itself to be the catalyst needed to deliver to labs the quality samples required for accurate COVID-19 detection.”
How It Works
- Individuals looking to purchase this EUA authorized saliva-based at home COVID-19 test should contact their doctor to order or visit spectrumsolution.com/COVID19-at-home for an authorized seller list.
- Patient arranges an appointment with a physician, which could be in person at the doctor’s office or via a telemedicine session.
- If physician determines the patient is a candidate for a COVID-19 test, the physician will prescribe the test and arrange for the test to be delivered to the patient.
- The patient will follow the simple saliva collection instructions in the kit on how to supply their saliva sample and how to return the sample to the processing lab through direct mail or drop off service.
- The lab will run the sample and report the results to the physician. The physician will then go over the results with the patient and determine the next course of action.

© Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
About Spectrum Solutions & Spectrum DNA
Headquartered in Salt Lake City, Utah, Spectrum Solutions and its medical device and services division, Spectrum DNA, focus their concentrated industry expertise on engineering innovative end-to-end solutions for both clinical diagnostic projects and commercial product plans. A single-source provider of on-site medical device manufacturing, custom packaging, kitting, and direct-to-consumer fulfillment. Our biosample collection devices, patented technologies, and dedicated services easily deliver measurable process optimization, unprecedented efficiency, and unmatched global scalability. For more information, please visit spectrumsolution.com/SDNA.

©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Headquartered in Salt Lake City, Utah, Spectrum Solutions and its medical device and services division, Spectrum DNA, focus their concentrated industry expertise on engineering innovative end-to-end solutions for both clinical diagnostic projects and commercial product plans. A single-source provider of on-site medical device manufacturing, custom packaging, kitting, and direct-to-consumer fulfillment. Our biosample collection devices, patented technologies, and dedicated services easily deliver measurable process optimization, unprecedented efficiency, and unmatched global scalability. For more information, please visit spectrumsolution.com/SDNA.

©Spectrum Solutions™ | Photo Credit: Leslie Titus Bryant
Spectrum
in the News
Spectrum
in the News
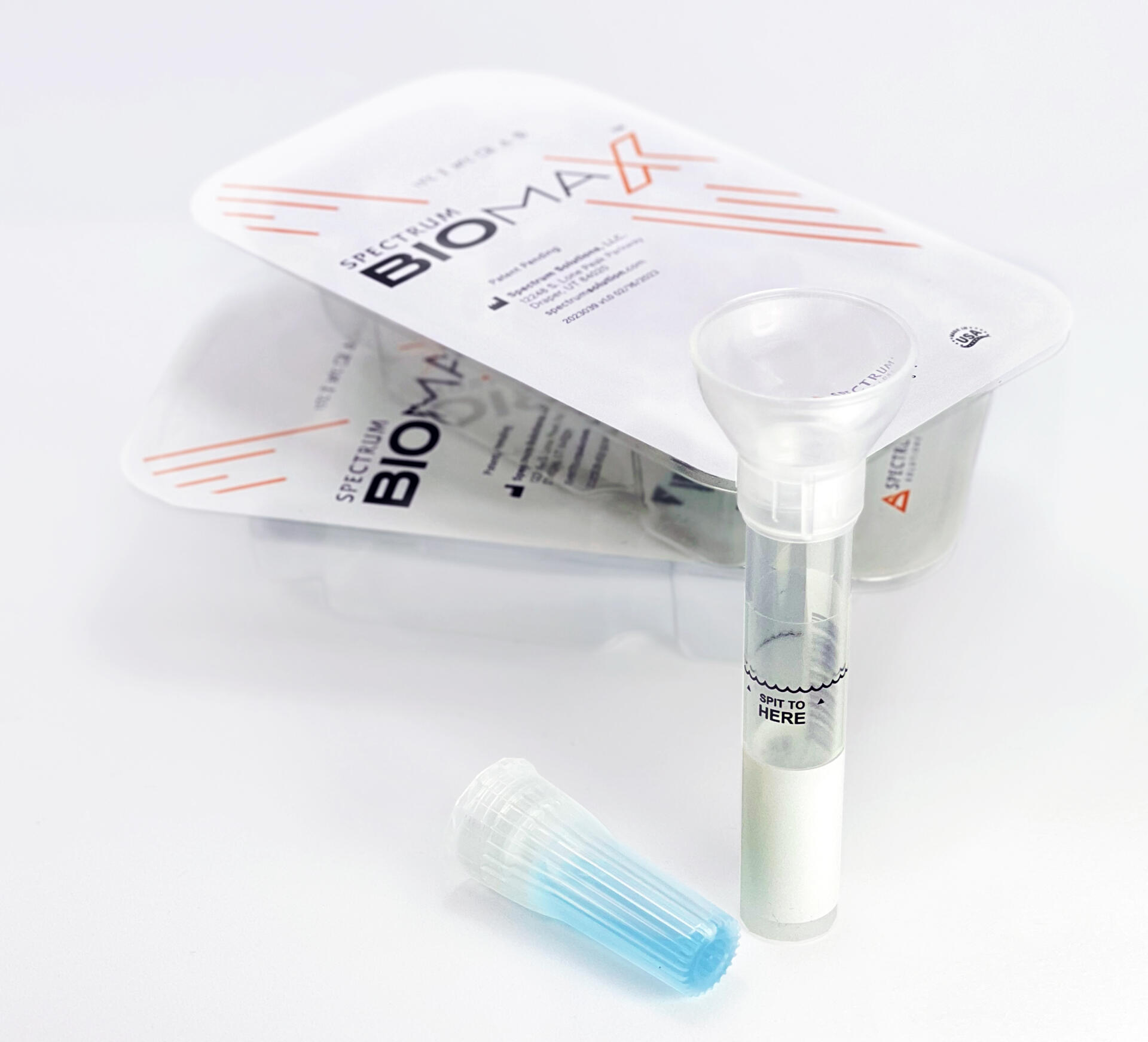
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.

