Innovating Sample Collection, Advancing Saliva Testing Applications
Easily Expanding Testing Access to More Patient Populations Worldwide
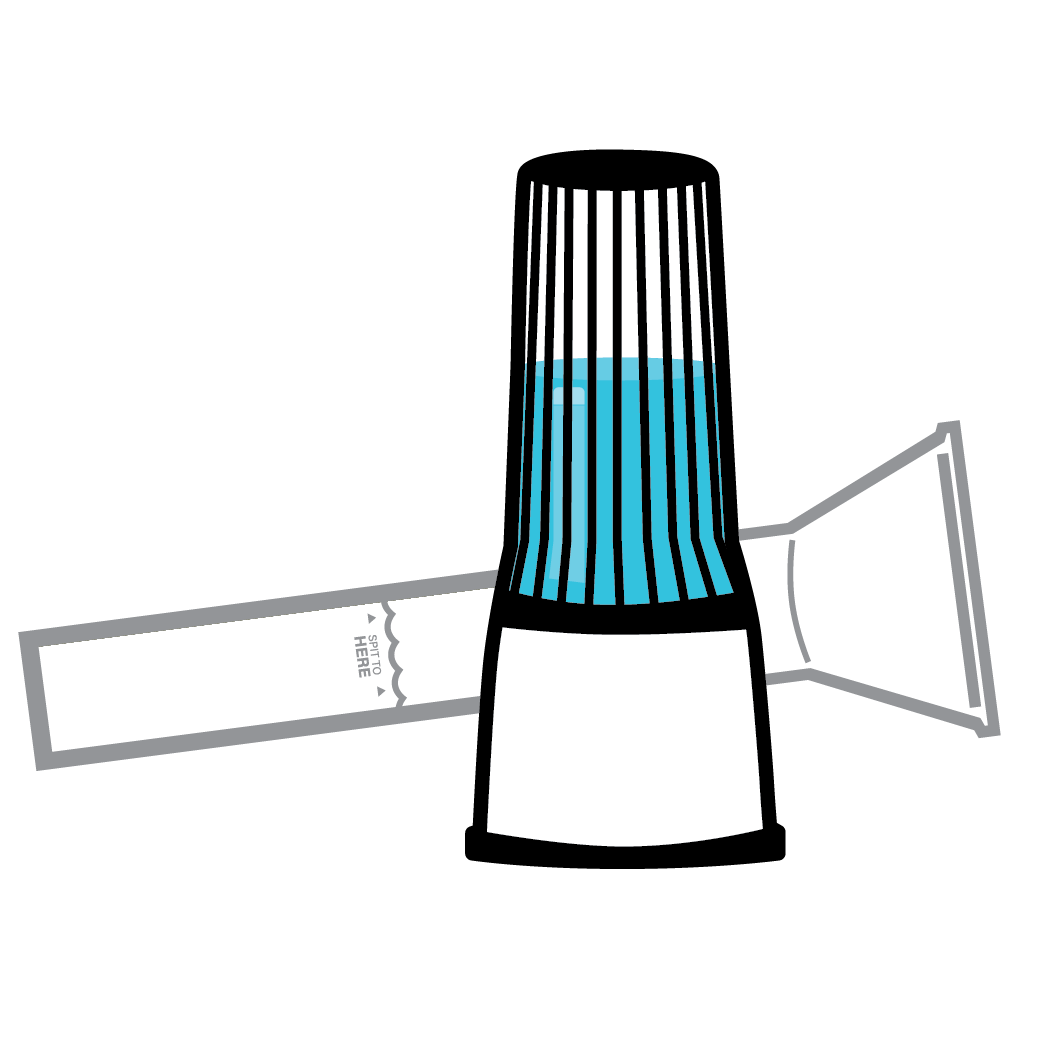
How you collect saliva makes a big diagnostic difference. Choosing the wrong saliva collection device can introduce and increase critical failure points. To incorporate the use of saliva into molecular testing protocols or implement actual real-world mass-testing in scalable scenarios it requires a studied, proven, validated, and supported system designed to increase test precision, sensitivity, and assay reproducibility.
The BioMAX™ family of specimen collection devices have been and continues to be, engineered to increase process simplicity and lead the clinical testing market in diagnostics and research applications. From efficient onsite device manufacturing for lower device costs to minimizing and eliminating unneeded process steps, saving valuable time. Compared to other saliva collection devices on the market, the BioMAX™ delivers an upgraded user experience with enhanced patient safety measures, tamper-evident packaging, and a patented buffer for preserving post-collection analyte integrity at ambient temperatures from weeks to years. For us, innovation is just “part of the solution.”
Sample self-collection using the BioMAX™ CV3 patented buffer provides 10-second in-device live viral neutralization for COVID-19, Flu A, Flu B, and RSV at ambient temperatures, mitigating any risk of unnecessary exposure for patients and all downstream processes.
Spectrum’s BioMAX™ saliva collection systems ship with verified, unique barcode serialization for unique biosample identification. Additionally, customized secondary packaging, clinical kitting, and direct-to-patient fulfillment options are available to solve any special project or testing workflow requirements.
Click here to learn more about the BioMAX™ SDNA-1000 Class II Medical Device FDA Clearance or here for the first saliva collection device to be authorized for COVID-19 testing.
Innovating Sample Collection, Advancing Saliva Testing Applications
Easily Expanding Testing Access to More Patient Populations Worldwide
How you collect saliva makes a big diagnostic difference. Choosing the wrong saliva collection device can introduce and increase critical failure points. To incorporate the use of saliva into molecular testing protocols or implement actual real-world mass-testing in scalable scenarios it requires a studied, proven, validated, and supported system designed to increase test precision, sensitivity, and assay reproducibility.
The BioMAX™ family of specimen collection devices have been and continues to be, engineered to increase process simplicity and lead the clinical testing market in diagnostics and research applications. From efficient onsite device manufacturing for lower device costs to minimizing and eliminating unneeded process steps, saving valuable time. Compared to other saliva collection devices on the market, the BioMAX™ delivers an upgraded user experience with enhanced patient safety measures, tamper-evident packaging, and a patented buffer for preserving post-collection analyte integrity at ambient temperatures from weeks to years. For us, innovation is just “part of the solution.”
Sample self-collection using the BioMAX™ CV3 patented buffer provides 10-second in-device live viral neutralization for COVID-19, Flu A, Flu B, and RSV at ambient temperatures, mitigating any risk of unnecessary exposure for patients and all downstream processes.
Spectrum’s BioMAX™ saliva collection systems ship with verified, unique barcode serialization for unique biosample identification. Additionally, customized secondary packaging, clinical kitting, and direct-to-patient fulfillment options are available to solve any special project or testing workflow requirements.
Click here to learn more about the BioMAX™ SDNA-1000 Class II Medical Device FDA Clearance or here for the first saliva collection device to be authorized for COVID-19 testing.
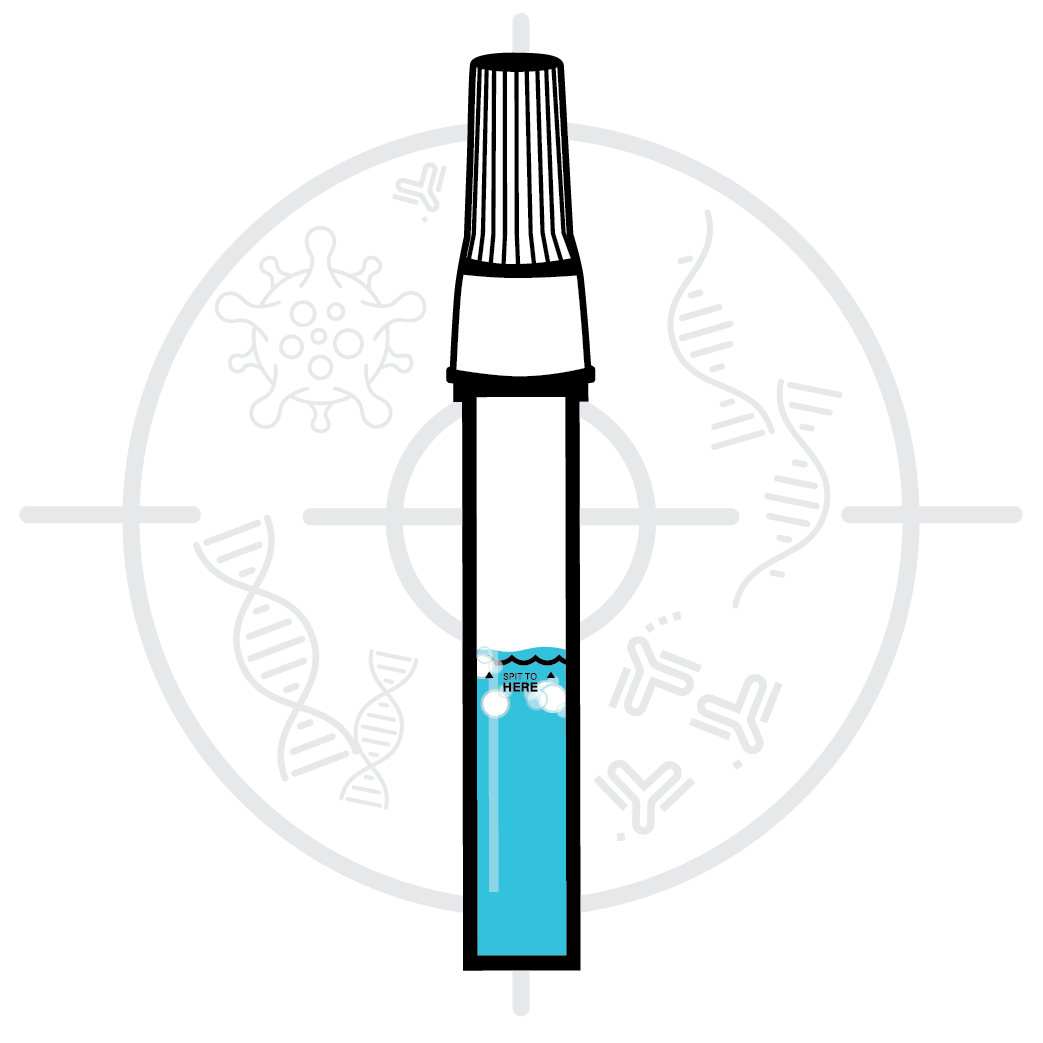
Designed to Eliminate
Self-Collection Errors
User error from the self-collection of saliva samples is one of the most common points of test failure.
Instead of another band-aid, we designed a solution.
Do not eat, drink, chew gum, or smoke at least 30 minutes before collecting your saliva.
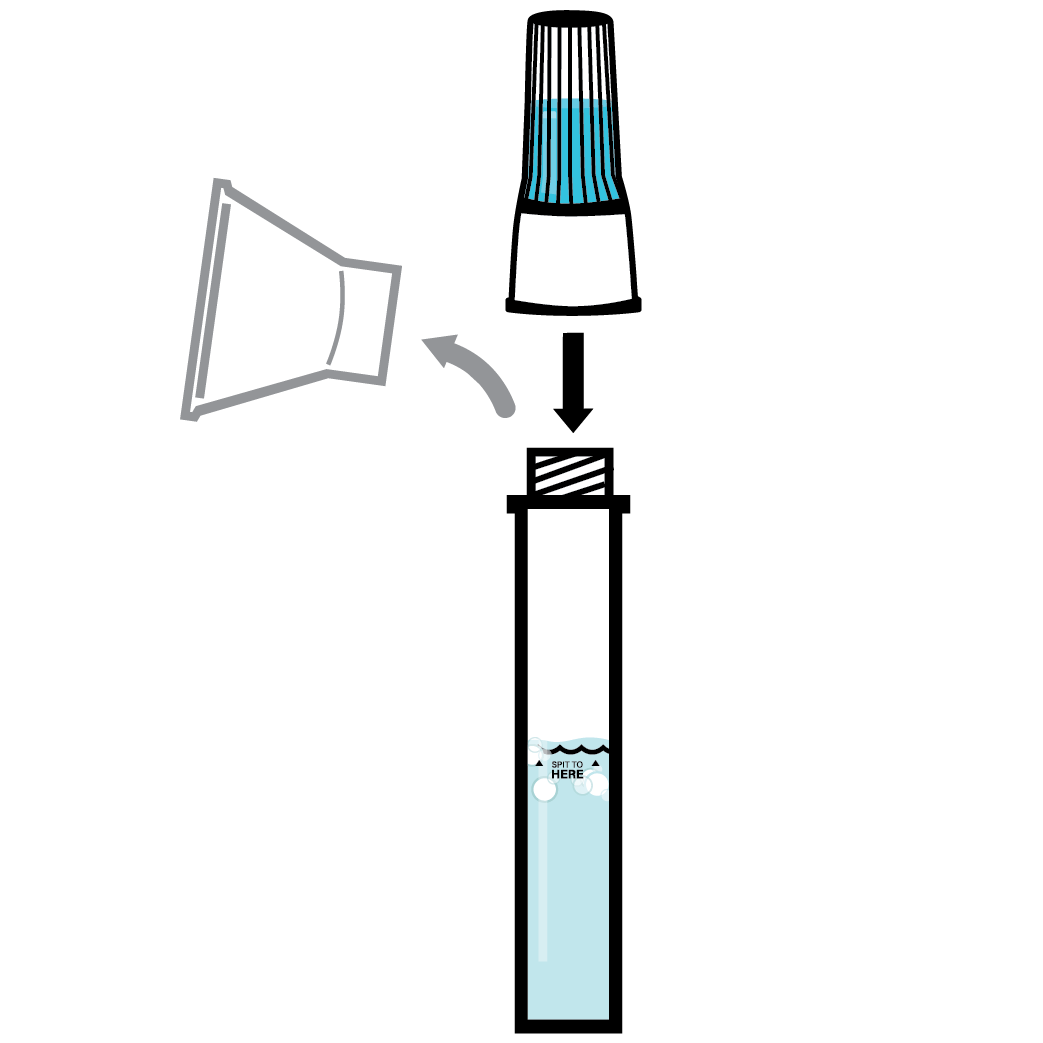
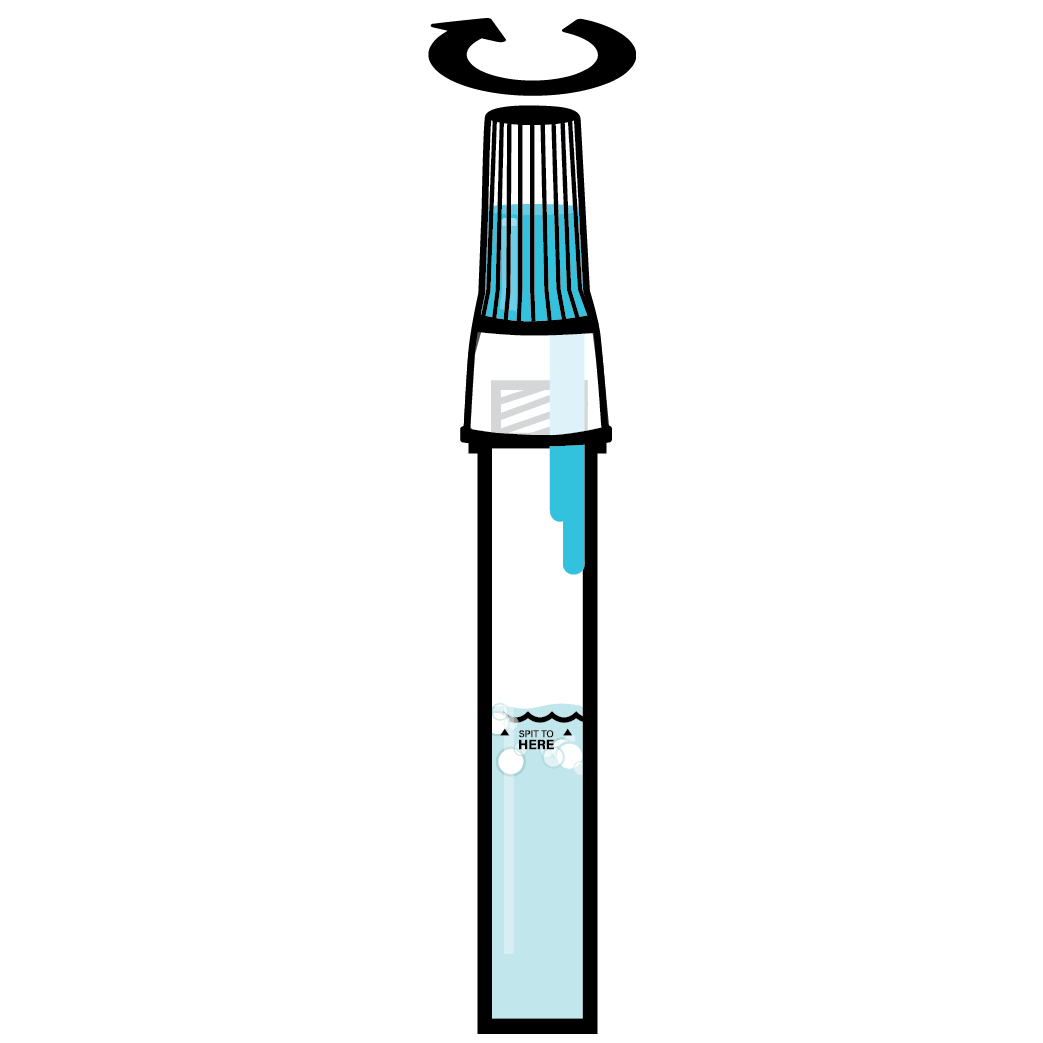
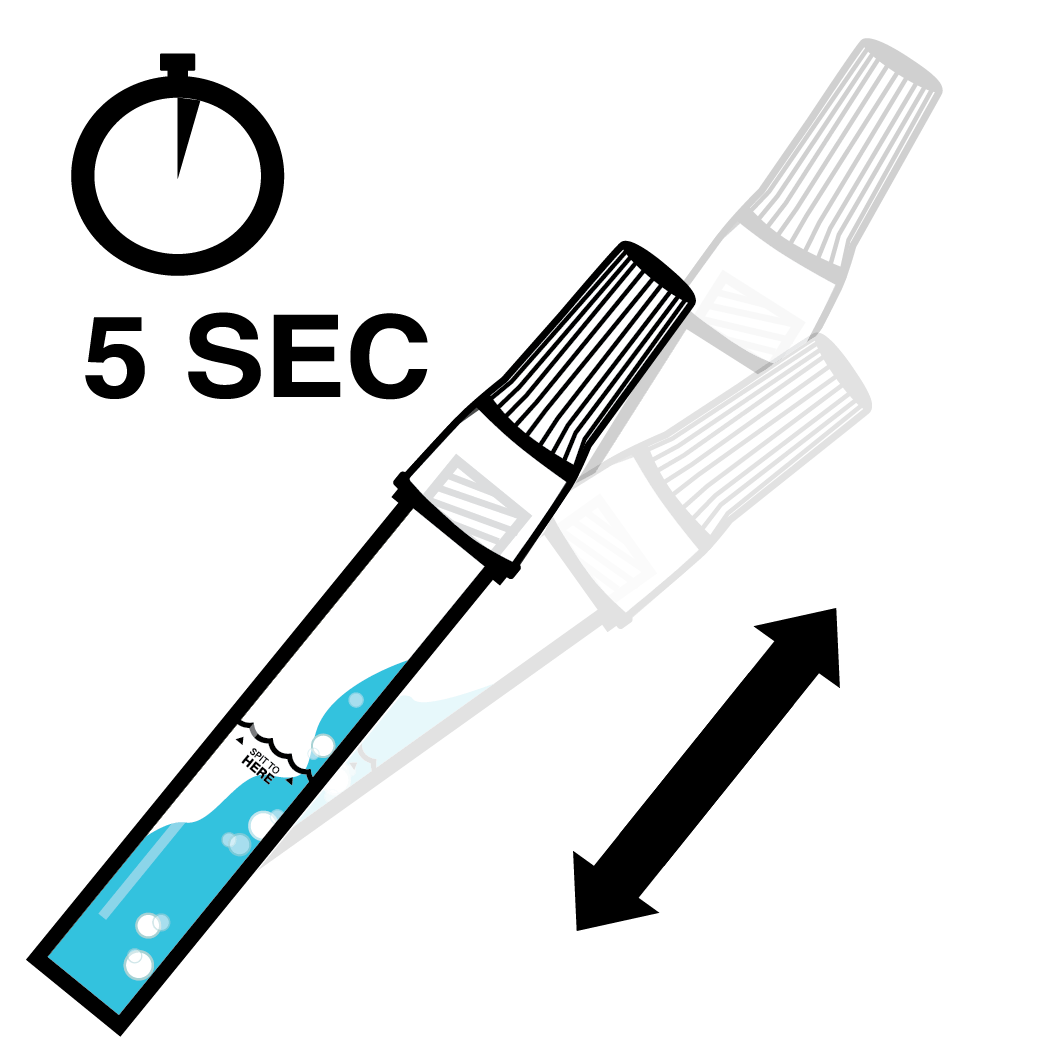
Designed to Eliminate Self-Collection Errors
User error from the self-collection of saliva samples is one of the most common points of test failure.
Instead of another band-aid, we designed a solution.
Do not eat, drink, chew gum, or smoke at least 30 minutes before collecting your saliva.




COVID-19 PCR
Saliva Testing
How you collect, preserve, and transport saliva is pivotal. The simplicity and effectiveness of Spectrum’s saliva collection devices have delivered a new standard of care for patients and laboratories, resulting in the large-scale adoption of saliva to drive screening, early detection, diagnosing, and the monitoring of disease.
- First FDA Authorized COVID-19 saliva testing solution
- First FDA Authorized at-home saliva collection device for COVID-19 testing
- Identify infections at their earliest stage before the onset of symptoms
- In-device 100% inactivation of the live virus within the device at ambient temps
- Maintains critical biosample consistency
- 99.998% testing accuracy & more sensitive
- No temperature-controlled storage or sample transport
- Post-collection stability with no degradation in sample efficacy
- Process multiple tests from a single collected sample
- Eliminate the need for UN3373 shipping designation
- Supported system and validated testing process
- At-home and direct-to-patient fulfillment
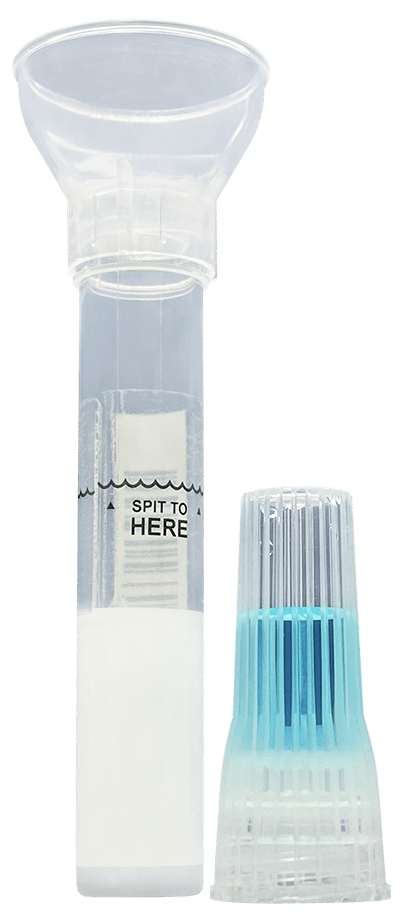
How-To Product
Video Tour
Huge amounts of innovative engineering have been poured into the development of our SDNA saliva collection device. Today its ease of use has been rewriting specimen collection protocols across the nation and worldwide. The intuitive design and minimal steps are a welcomed answer to those seeing clinical study and project failures due to repeat bio-sample self-collection errors from the lack of viable options.
How-To Product
Video Tour
Huge amounts of innovative engineering have been poured into the development of our SDNA saliva collection device. Today its ease of use has been rewriting specimen collection protocols across the nation and worldwide. The intuitive design and minimal steps are a welcomed answer to those seeing clinical study and project failures due to repeat bio-sample self-collection errors from the lack of viable options.
What does technically
superior mean?
What does technically superior mean?
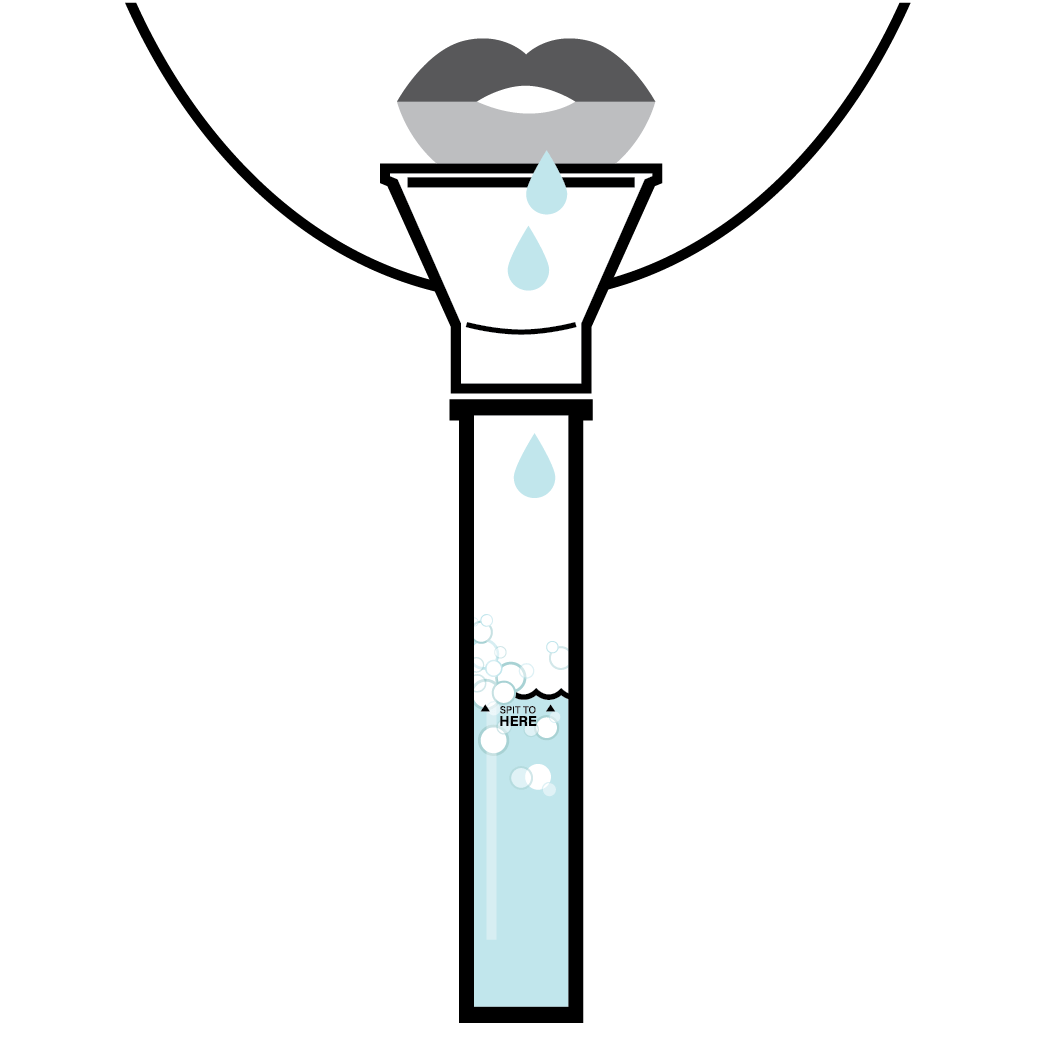
Solution for Self-Collection Errors
Proof-PointSignificantly Reduce Most Common Sources of User Error
Spectrum’s whole saliva collection device addresses the three most common sources of user error, with easy-to-understand instructions and intuitive device design.
[User study underway – data available Dec. 2021 ]
Process 8,000 COVID-19 Tests/day (+variants)
Proof-PointThermo Fisher's Amplitude Solution + the SDNA-1000 expand testing capacity with minimal time, equipment, & staff
“SDNA saliva collection devices are self-contained systems that provide sample consistency and long-term stability while protecting DNA and RNA transcripts post-collection to ensure accurate test results”
Is it COVID-19? The Flu? H1N1? or...
Proof-PointAt-Home Saliva Collection Offers Easy Answers
Over-engineered on purpose to solve genetic testing and molecular diagnostic industry pain-points, the SDNA-1000 is leading the way to a new era of at-home biosample self-collection for the diagnosis of different viral infections.
[White paper coming soon]
Saliva is a Better Biosample for Detecting Infection
Proof-PointStudies Echo Rutgers Significant Saliva Findings
Understanding the breakthrough discovery saliva has delivered goes far beyond expanding COVID-19 testing options. It offers the safest & most robust biomaterial for detecting viral infections and mitigates any risk of infection throughout the entire testing process.
Saliva Sample Viability Transport Study
Proof-PointTested in extreme hot and cold temperatures
FDA Expands current EUA to include the at-home collection of saliva samples for COVID-19 testing. Direct-to-patient at-home collection adds testing complexity and risks sample consistency, sample viability, and sample stability. See the stats and specifics behind the FDA EUA authorization.