BIOWORLD:
In Attempt to Quell a Pandemic U.K. Launches Free COVID-19 Testing Program
Nuala Moran, BioWorld
January 12, 2021
| Nuala Moran, BioWorld January 12, 2021 |
|---|
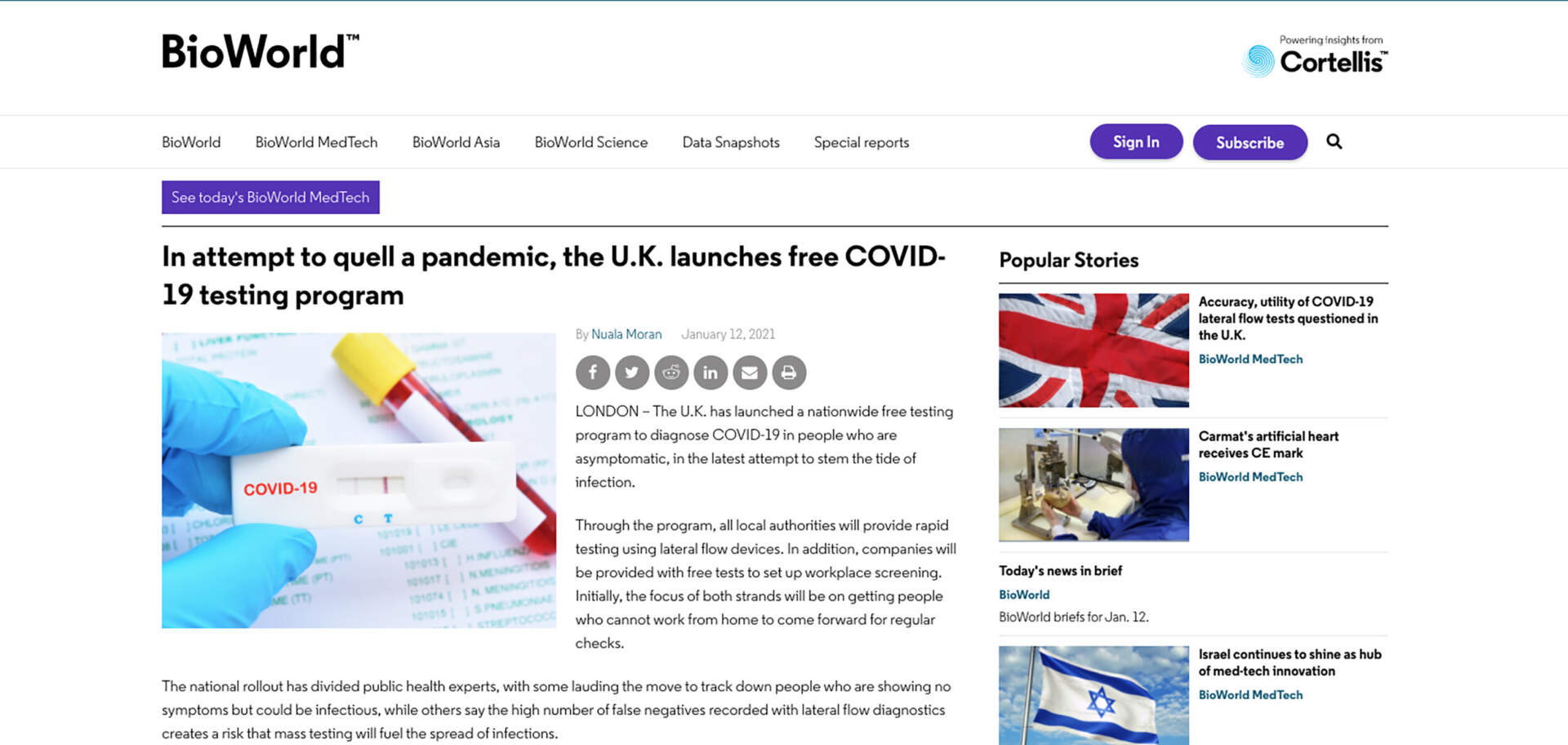
LONDON – The U.K. has launched a nationwide free testing program to diagnose COVID-19 in people who are asymptomatic, in the latest attempt to stem the tide of infection.
Through the program, all local authorities will provide rapid testing using lateral flow devices. In addition, companies will be provided with free tests to set up workplace screening. Initially, the focus of both strands will be on getting people who cannot work from home to come forward for regular checks.
The national rollout has divided public health experts, with some lauding the move to track down people who are showing no symptoms but could be infectious, while others say the high number of false negatives recorded with lateral flow diagnostics creates a risk that mass testing will fuel the spread of infections.
Around one-third of people who get infected with SARS-CoV-2 show no symptoms but may be infectious.
The decision to scale up asymptomatic testing follows a number of pilots, most notably one in the city of Liverpool, from which first results were published at the end of last month. The data indicate the sensitivity of point of care lateral flow testing is less than 40%, compared to laboratory-processed PCR tests. However, lateral flow devices did pick up 1,300 cases of COVID-19 in people who would not have been eligible for PCR testing because they had no symptoms.
Liverpool and other pilots used a lateral flow device supplied by Innova Medical Group Inc., of Monrovia, Calif., in a US$138 million contract with the U.K. government. The government has now announced a second approved supplier Surescreen Diagnostics Ltd., of Derby, U.K., and said it has placed an initial order for 2 million of its tests, to be delivered by January 15.
In a move it says will help expand access to PCR testing in support of the U.K. national screening program, Spectrum Solutions LLC of Salt Lake City, Utah, which developed the first FDA approved saliva collection device, reported it has signed a U.K distribution agreement for the product.
In addition to being easier to take a saliva sample than a nasal swab, the Spectrum SDNA-1000 collection device inactivates any live virus, while keeping it viable for analyzing for at least ten days from the point of collection. That will provide greater flexibility to manage workloads and mean the entire installed PCR base in the U.K., in both the public and private establishments, can be fully and efficiently utilized in mass testing, according to Spectrum’s partner, Steribottle Ltd. of London, a company which is best known for manufacturing disposable baby feeding bottles.
Separately, Spectrum announced it has received CE marking to sell SDNA-1000 in Europe.

BIOWORLD:
In Attempt to Quell a Pandemic U.K. Launches Free COVID-19 Testing Program
Nuala Moran, BioWorld
January 12, 2021
| Nuala Moran, BioWorld January 12, 2021 |
|---|
LONDON – The U.K. has launched a nationwide free testing program to diagnose COVID-19 in people who are asymptomatic, in the latest attempt to stem the tide of infection.
Through the program, all local authorities will provide rapid testing using lateral flow devices. In addition, companies will be provided with free tests to set up workplace screening. Initially, the focus of both strands will be on getting people who cannot work from home to come forward for regular checks.
The national rollout has divided public health experts, with some lauding the move to track down people who are showing no symptoms but could be infectious, while others say the high number of false negatives recorded with lateral flow diagnostics creates a risk that mass testing will fuel the spread of infections.
Around one-third of people who get infected with SARS-CoV-2 show no symptoms but may be infectious.
The decision to scale up asymptomatic testing follows a number of pilots, most notably one in the city of Liverpool, from which first results were published at the end of last month. The data indicate the sensitivity of point of care lateral flow testing is less than 40%, compared to laboratory-processed PCR tests. However, lateral flow devices did pick up 1,300 cases of COVID-19 in people who would not have been eligible for PCR testing because they had no symptoms.
Liverpool and other pilots used a lateral flow device supplied by Innova Medical Group Inc., of Monrovia, Calif., in a US$138 million contract with the U.K. government. The government has now announced a second approved supplier Surescreen Diagnostics Ltd., of Derby, U.K., and said it has placed an initial order for 2 million of its tests, to be delivered by January 15.
In a move it says will help expand access to PCR testing in support of the U.K. national screening program, Spectrum Solutions LLC of Salt Lake City, Utah, which developed the first FDA approved saliva collection device, reported it has signed a U.K distribution agreement for the product.
In addition to being easier to take a saliva sample than a nasal swab, the Spectrum SDNA-1000 collection device inactivates any live virus, while keeping it viable for analyzing for at least ten days from the point of collection. That will provide greater flexibility to manage workloads and mean the entire installed PCR base in the U.K., in both the public and private establishments, can be fully and efficiently utilized in mass testing, according to Spectrum’s partner, Steribottle Ltd. of London, a company which is best known for manufacturing disposable baby feeding bottles.
Separately, Spectrum announced it has received CE marking to sell SDNA-1000 in Europe.

Argument Over Lateral Flow Amplifying
Based on experience to date with testing asymptomatic people with a lateral flow device in Liverpool, Iain Buchan, professor of public health and clinical informatics at Liverpool University, who is leading the program in the city said, “There is an opportunity to target lateral flow test capacity at twice-weekly testing for those who have to go out to work.”
Similarly, Jose Vazquez-Boland, chair of infectious diseases at Edinburgh University medical school said mass, systematic, regular screening of the population, “is the only workable strategy to control the spread of this highly transmissible virus.”
The focus to date on testing only people showing symptoms has been “short-sighted,” addressing “the tip of the iceberg, rather than tackling the much wider submerged base of asymptomatic carriers responsible for the silent spread of the coronavirus,” said Vazquez-Boland.
James Gill of Warwick University Medical School agreed. Asymptomatic COVID-19 positive people are thought to be infectious for a shorter period of time, but “identifying and isolating such individuals will be a turning point, as we can hope and expect their identification to reduce new case trajectories,” he said.
Ranged against these views, Alexander Edwards, associate professor of biomedical technology in the school of pharmacy at Reading University, noted COVID-19 tests are designed, checked, and approved for symptomatic testing. “Manufacturers typically prove their products work by testing samples from symptomatic infected patients. The accuracy drops significantly when used with larger groups of symptomatic people,” he said.
Accuracy also often drops when used in the community, rather than administered by trained experts. “It’s not clear how community testing centers can even check how accurate their testing service is,” Edwards said.
One of the most scathing critics of using lateral flow tests for population screening is Jon Deeks, head of the biostatistics, evidence synthesis, and test evaluation research group at Birmingham University. He said the government’s plan to roll out mass testing, “brings a real risk that it will increase rather than decrease the spread of COVID.”
Deeks said negative test results lead people to relax and change their behavior. “With the Innova test this is false reassurance – there are reports that false negatives have led people to ignore symptoms, leading to disease spread,” he said.
This was echoed by Angela Raffle, consultant in public health at Bristol University Medical School. For her, the news of further rollout of lateral flow testing is “very worrying.” Any benefit from finding symptomless cases will be outweighed by the many more infectious cases that are missed. “Already, outbreaks are known to have occurred because people have been falsely reassured by a negative lateral flow test, leading them to attend work whilst having symptoms,” she said.
Argument Over Lateral Flow Amplifying
Based on experience to date with testing asymptomatic people with a lateral flow device in Liverpool, Iain Buchan, professor of public health and clinical informatics at Liverpool University, who is leading the program in the city said, “There is an opportunity to target lateral flow test capacity at twice-weekly testing for those who have to go out to work.”
Similarly, Jose Vazquez-Boland, chair of infectious diseases at Edinburgh University medical school said mass, systematic, regular screening of the population, “is the only workable strategy to control the spread of this highly transmissible virus.”
The focus to date on testing only people showing symptoms has been “short-sighted,” addressing “the tip of the iceberg, rather than tackling the much wider submerged base of asymptomatic carriers responsible for the silent spread of the coronavirus,” said Vazquez-Boland.
James Gill of Warwick University Medical School agreed. Asymptomatic COVID-19 positive people are thought to be infectious for a shorter period of time, but “identifying and isolating such individuals will be a turning point, as we can hope and expect their identification to reduce new case trajectories,” he said.
Ranged against these views, Alexander Edwards, associate professor of biomedical technology in the school of pharmacy at Reading University, noted COVID-19 tests are designed, checked, and approved for symptomatic testing. “Manufacturers typically prove their products work by testing samples from symptomatic infected patients. The accuracy drops significantly when used with larger groups of symptomatic people,” he said.
Accuracy also often drops when used in the community, rather than administered by trained experts. “It’s not clear how community testing centers can even check how accurate their testing service is,” Edwards said.
One of the most scathing critics of using lateral flow tests for population screening is Jon Deeks, head of the biostatistics, evidence synthesis, and test evaluation research group at Birmingham University. He said the government’s plan to roll out mass testing, “brings a real risk that it will increase rather than decrease the spread of COVID.”
Deeks said negative test results lead people to relax and change their behavior. “With the Innova test this is false reassurance – there are reports that false negatives have led people to ignore symptoms, leading to disease spread,” he said.
This was echoed by Angela Raffle, consultant in public health at Bristol University Medical School. For her, the news of further rollout of lateral flow testing is “very worrying.” Any benefit from finding symptomless cases will be outweighed by the many more infectious cases that are missed. “Already, outbreaks are known to have occurred because people have been falsely reassured by a negative lateral flow test, leading them to attend work whilst having symptoms,” she said.

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB

Bringing Baseball Back!
How you collect saliva makes a big difference
Increase workplace safety and build team confidence with simple and safe repeat testing programs supporting 100% accurate early detection and easy direct-to-user at-home options. Just ask the Major League Baseball. See how our saliva collection system is credited for “bringing baseball back” and making Salt Lake “the league’s most important city”.
™/© 2020 MLB
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
About Spectrum Solutions®
Headquartered in Salt Lake City, Utah, Spectrum Solutions is dedicated to empowering complete wellness and bridging the gap between science and innovative healthcare solutions. Our stand-alone and fully integrated test-to-treat solutions support molecular diagnostics and DTC testing applications, advancing product development and accelerating go-to-market applications. Our single-source, end-to-end capabilities include a CAP/CLIA accredited molecular diagnostic laboratory, onsite compounding pharmacy, medical and non-medical product development, manufacturing, and fulfillment.
![]()
 Spectrum Corporate Spokesman
Spectrum Corporate Spokesman
Leslie Titus Bryant
Head of Marketing & Brand
admin@spectrumsolution.com
 Media Contact
Media Contact
Tim Rush, Springboard5
801-208-1100
tim.rush@springboard5.com
Spectrum
in the News
Spectrum
in the News
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.
Noninvasive
Saliva Diagnostics
This changes everything!
Saliva analysis looks at the cellular level, the biologically active compounds, making it a true representative of what is clinically relevant. Engineered to lead the saliva collection industry, the BioMAX™ delivers the safest and most robust biosample for the earliest detection and diagnosis of disease and infection.
Since 2020 and the COVID-19 pandemic, Spectrum’s saliva collection system not only introduced, it continues to expand the molecular diagnostics industry and its understanding of the opportunities saliva offers patients, providers, and laboratories.

Outside-of-the-Box Thinking, Inside-of-the-Box Innovation
Anywhere from customized testing solutions to new medical science product innovations–we’re here to help.